Microencapsulation and nanowarming enables vitrification cryopreservation of mouse preantral follicles
- PMID: 36522314
- PMCID: PMC9755531
- DOI: 10.1038/s41467-022-34549-2
Microencapsulation and nanowarming enables vitrification cryopreservation of mouse preantral follicles
Abstract
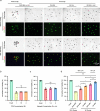
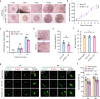
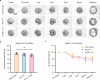
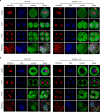
Preantral follicles are often used as models for cryopreservation and in vitro culture due to their easy availability. As a promising approach for mammalian fertility preservation, vitrification of preantral follicles requires high concentrations of highly toxic penetrating cryoprotective agents (up to 6 M). Here, we accomplish low-concentration-penetrating cryoprotective agent (1.5 M) vitrification of mouse preantral follicles encapsulated in hydrogel by nanowarming. We find that compared with conventional water bath warming, the viability of preantral follicles is increased by 33%. Moreover, the cavity formation rate of preantral follicles after in vitro culture is comparable to the control group without vitrification. Furthermore, the percentage of MII oocytes developed from the vitrified follicles, and the birth rate of offspring following in vitro fertilization and embryo transfer are also similar to the control group. Our results provide a step towards nontoxic vitrification by utilizing the synergistic cryoprotection effect of microencapsulation and nanowarming.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Breast cancer statistics. Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

