Characterization of pulmonary vascular remodeling and MicroRNA-126-targets in COPD-pulmonary hypertension
- PMID: 36522710
- PMCID: PMC9756782
- DOI: 10.1186/s12931-022-02267-4
Characterization of pulmonary vascular remodeling and MicroRNA-126-targets in COPD-pulmonary hypertension
Abstract
Background: Despite causing increased morbidity and mortality, pulmonary hypertension (PH) in chronic obstructive pulmonary disease (COPD) patients (COPD-PH) lacks treatment, due to incomplete understanding of its pathogenesis. Hypertrophy of pulmonary arterial walls and pruning of the microvasculature with loss of capillary beds are known features of pulmonary vascular remodeling in COPD. The remodeling features of pulmonary medium- and smaller vessels in COPD-PH lungs are less well described and may be linked to maladaptation of endothelial cells to chronic cigarette smoking (CS). MicroRNA-126 (miR126), a master regulator of endothelial cell fate, has divergent functions that are vessel-size specific, supporting the survival of large vessel endothelial cells and inhibiting the proliferation of microvascular endothelial cells. Since CS decreases miR126 in microvascular lung endothelial cells, we set out to characterize the remodeling by pulmonary vascular size in COPD-PH and its relationship with miR126 in COPD and COPD-PH lungs.
Methods: Deidentified lung tissue was obtained from individuals with COPD with and without PH and from non-diseased non-smokers and smokers. Pulmonary artery remodeling was assessed by ⍺-smooth muscle actin (SMA) abundance via immunohistochemistry and analyzed by pulmonary artery size. miR126 and miR126-target abundance were quantified by qPCR. The expression levels of ceramide, ADAM9, and endothelial cell marker CD31 were assessed by immunofluorescence.
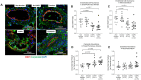
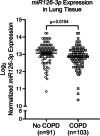
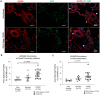
Results: Pulmonary arteries from COPD and COPD-PH lungs had significantly increased SMA abundance compared to non-COPD lungs, especially in small pulmonary arteries and the lung microvasculature. This was accompanied by significantly fewer endothelial cell markers and increased pro-apoptotic ceramide abundance. miR126 expression was significantly decreased in lungs of COPD individuals. Of the targets tested (SPRED1, VEGF, LAT1, ADAM9), lung miR126 most significantly inversely correlated with ADAM9 expression. Compared to controls, ADAM9 was significantly increased in COPD and COPD-PH lungs, predominantly in small pulmonary arteries and lung microvasculature.
Conclusion: Both COPD and COPD-PH lungs exhibited significant remodeling of the pulmonary vascular bed of small and microvascular size, suggesting these changes may occur before or independent of the clinical development of PH. Decreased miR126 expression with reciprocal increase in ADAM9 may regulate endothelial cell survival and vascular remodeling in small pulmonary arteries and lung microvasculature in COPD and COPD-PH.
Keywords: ADAM9; Chronic obstructive pulmonary disease; Emphysema; Endothelial cells; Group 3 pulmonary hypertension; Pulmonary vascular remodeling; Smoking; microRNA-126.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

