Human umbilical cord platelet-rich plasma to treat endometrial pathologies: methodology, composition and pre-clinical models
- PMID: 36523324
- PMCID: PMC9747096
- DOI: 10.1093/hropen/hoac053
Human umbilical cord platelet-rich plasma to treat endometrial pathologies: methodology, composition and pre-clinical models
Abstract
Study question: Can human umbilical cord platelet-rich plasma (hUC-PRP) efficiently treat endometrial damage and restore fertility in a preclinical murine model?
Summary answer: Local application of hUC-PRP promotes tissue regeneration and fertility restoration in a murine model of Asherman syndrome and endometrial atrophy (AS/EA).
What is known already: AS/EA are well-described endometrial pathologies that cause infertility; however, there are currently no gold-standard treatments available. Recent reports have described the successful use of human platelet-rich plasma in reproductive medicine, and its regenerative potential is further enhanced using hUC-PRP, due to the ample growth factors and reduced pro-inflammatory cytokines in the latter.
Study design size duration: hUC-PRP (n = 3) was processed, characterized and delivered locally to endometrial damage in a murine model (n = 50). The hUC-PRP was either used alone or loaded into a decellularized porcine endometrium-derived extracellular matrix (EndoECM) hydrogel; endometrial regeneration, fertility outcomes and immunocompatibility were evaluated 2 weeks following treatment administration.
Participants/materials setting methods: Umbilical cord blood was obtained from women in childbirth. Endometrial damage (mimicking AS/EA) was induced using ethanol in 8-week-old C57BL/6 mice, and treated with the most concentrated hUC-PRP sample 4 days later. Characterization of hUC-PRP and immunotolerance was carried out with multiplex technology, while uterine samples were analyzed by immunohistochemistry and quantitative PCR. The number of embryos and their morphology was determined visually.
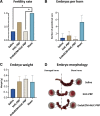
Main results and the role of chance: Platelet density was enhanced 3-fold in hUC-PRP compared to that in hUC blood (P < 0.05). hUC-PRP was enriched with growth factors related to tissue regeneration (i.e. hepatocyte growth factor, platelet-derived growth factor-BB and epidermal growth factor), which were released constantly (in vitro) when hUC-PRP was loaded into EndoECM. Both treatments (hUC-PRP alone and hUC-PRP with EndoECM) were immunotolerated and caused significantly regeneration of the damaged endometrium, evidenced by increased endometrial area, neoangiogenesis, cell proliferation and gland density and lower collagen deposition with respect to non-treated uterine horns (P < 0.05). Additionally, we detected augmented gene expression of Akt1, VEGF and Ang, which are involved in regenerative and proliferation pathways. Finally, hUC-PRP treatment restored pregnancy rates in the mouse model.
Large scale data: N/A.
Limitations reasons for caution: This proof-of-concept pilot study was based on a murine model of endometrial damage and the use of EndoECM requires further validation prior to clinical implementation for women affected by AS/EA.
Wider implications of the findings: The local administration of hUC-PRP has high impact and is immunotolerated in a murine model of AS/EA, as has been reported in other tissues, making it a promising candidate for heterologous treatment of these endometrial pathologies.
Study funding/competing interests: This study was supported by the Ministerio de Ciencia, Innovación y Universidades; Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital, Generalitat Valenciana; and Instituto de Salud Carlos III. The authors do not have any conflicts of interest to declare.
Keywords: endometrium; extracellular matrix hydrogel; human umbilical cord; platelet-rich plasma; regenerative medicine.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures





References
-
- Berney M, McCarroll P, Glynn L, Lenehan B.. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci 2020;190:1021–1025. - PubMed
-
- Buigues A, Marchante M, de Miguel-Gómez L, Martinez J, Cervelló I, Pellicer A, Herraiz S.. Stem cell-secreted factor therapy regenerates the ovarian niche and rescues follicles. Am J Obstet Gynecol 2021;225:65.e1–65.e14. - PubMed
-
- Buzzi M, Versura P, Grigolo B, Cavallo C, Terzi A, Pellegrini M, Giannaccare G, Randi V, Campos EC.. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus Apher Sci 2018;57:549–555. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
