A pipeline for assembling low copy nuclear markers from plant genome skimming data for phylogenetic use
- PMID: 36523475
- PMCID: PMC9745922
- DOI: 10.7717/peerj.14525
A pipeline for assembling low copy nuclear markers from plant genome skimming data for phylogenetic use
Abstract
Background: Genome skimming is a popular method in plant phylogenomics that do not include a biased enrichment step, relying on random shallow sequencing of total genomic DNA. From these data the plastome is usually readily assembled and constitutes the bulk of phylogenetic information generated in these studies. Despite a few attempts to use genome skims to recover low copy nuclear loci for direct phylogenetic use, such endeavor remains neglected. Causes might include the trade-off between libraries with few reads and species with large genomes (i.e., missing data caused by low coverage), but also might relate to the lack of pipelines for data assembling.
Methods: A pipeline and its companion R package designed to automate the recovery of low copy nuclear markers from genome skimming libraries are presented. Additionally, a series of analyses aiming to evaluate the impact of key assembling parameters, reference selection and missing data are presented.
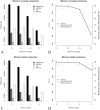
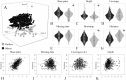
Results: A substantial amount of putative low copy nuclear loci was assembled and proved useful to base phylogenetic inference across the libraries tested (4 to 11 times more data than previously assembled plastomes from the same libraries).
Discussion: Critical aspects of assembling low copy nuclear markers from genome skims include the minimum coverage and depth of a sequence to be used. More stringent values of these parameters reduces the amount of assembled data and increases the relative amount of missing data, which can compromise phylogenetic inference, in turn relaxing the same parameters might increase sequence error. These issues are discussed in the text, and parameter tuning through multiple comparisons tracking their effects on support and congruence is highly recommended when using this pipeline. The skimmingLoci pipeline (https://github.com/mreginato/skimmingLoci) might stimulate the use of genome skims to recover nuclear loci for direct phylogenetic use, increasing the power of genome skimming data to resolve phylogenetic relationships, while reducing the amount of sequenced DNA that is commonly wasted.
Keywords: Genome skimming; High-throughput sequencing; Low copy; Mapping reads; Phylogenetics; Pipeline; R package; systematics.
© 2022 Reginato.
Conflict of interest statement
The author declares he has no competing interests.
Figures



References
-
- Besnard G, Bianconi ME, Hackel J, Manzi S, Vorontsova MS, Christin PA. Herbarium genomics retraces the origins of C4-specific carbonic anhydrase in Andropogoneae (Poaceae) Botany Letters. 2018;165(3–4):419–433. doi: 10.1080/23818107.2018.1469429. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

