Seasonal patterns of rhizosphere microorganisms suggest carbohydrate-degrading and nitrogen-fixing microbes contribute to the attribute of full-year shooting in woody bamboo Cephalostachyum pingbianense
- PMID: 36523824
- PMCID: PMC9745117
- DOI: 10.3389/fmicb.2022.1033293
Seasonal patterns of rhizosphere microorganisms suggest carbohydrate-degrading and nitrogen-fixing microbes contribute to the attribute of full-year shooting in woody bamboo Cephalostachyum pingbianense
Abstract
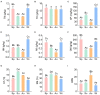
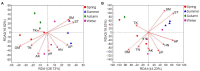
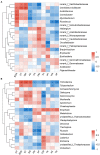
Compared with the ordinary single-season shooting among woody bamboos in Poaceae, the attribute of full-year shooting in Cephalostachyum pingbianense represents a unique shooting type or mechanism. Nevertheless, except for the overall physiological mechanism, the effect of ecological factors, especially soil microorganisms, on this full-year shooting characteristic remains unclear. In this study, 16S rRNA and ITS rRNA genes were sequenced using the Illumina platform. Our aims were to detect the seasonal changes in rhizospheric microbial communities of C. pingbianense and to discover the correlations of soil microbes with soil properties and bamboo shoot productivity. The results showed that seasonal change had no significant effect on bacterial alpha diversity, but significantly affected bacterial and fungal community structures as well as fungal richness. Among all soil properties examined, soil temperature, soil moisture and organic matter were the predominant factors affecting bacterial community diversity and structure. Soil temperature and soil moisture also significantly influenced fungal community structure, while available phosphorus had the greatest effect on fungal diversity. In each season, bacterial genera Acidothermus, Roseiarcus, and Bradyrhizobium, along with fungal genera Saitozyma, Mortierella, Trichoderma, etc., were dominant in bacterial and fungal communities, respectively. Bacterial community functions in four seasons were dominated by chemoheterotrophy, cellulolysis, and nitrogen fixation. Saprotrophic fungi occupied a high proportion in soil samples of all seasons. In addition, correlation analysis revealed that the bamboo shoot productivity was positively correlated with multiple microbial taxa involved in carbon and nitrogen cycles. It is proposed that highly abundant microbes involved in carbohydrate degradation and nitrogen fixation in the rhizosphere soil may contribute to the attribute of producing bamboo shoots all year round in C. pingbianense. This study is among the few cases revealing the connection between bamboo shooting characteristics and soil microorganisms, and provides new physiological and ecological insights into the forest management of woody bamboos.
Keywords: Cephalostachyumpingbianense; bamboo shooting; microbial function; rhizosphere microbe; seasonal variation.
Copyright © 2022 Li, Xia and Yang.
Figures




References
-
- Backer R., Rokem J. S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., et al. . (2018). Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 9:1473. doi: 10.3389/fpls.2018.01473, PMID: - DOI - PMC - PubMed
-
- Ballhausen M. B., De Boer W. (2016). The sapro-rhizosphere: carbon flow from saprotrophic fungi into fungus-feeding bacteria. Soil Biol. Biochem. 102, 14–17. doi: 10.1016/j.soilbio.2016.06.014 - DOI
LinkOut - more resources
Full Text Sources

