Aspartate α-decarboxylase a new therapeutic target in the fight against Helicobacter pylori infection
- PMID: 36523828
- PMCID: PMC9746714
- DOI: 10.3389/fmicb.2022.1019666
Aspartate α-decarboxylase a new therapeutic target in the fight against Helicobacter pylori infection
Abstract
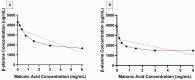
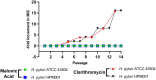
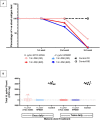
Effective eradication therapy for Helicobacter pylori is a worldwide demand. Aspartate α-decarboxylase (ADC) was reported as a drug target in H. pylori, in an in silico study, with malonic acid (MA) as its inhibitor. We evaluated eradicating H. pylori infection through ADC inhibition and the possibility of resistance development. MA binding to ADC was modeled via molecular docking. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of MA were determined against H. pylori ATCC 43504, and a clinical H. pylori isolate. To confirm selective ADC inhibition, we redetermined the MIC in the presence of products of the inhibited enzymatic pathway: β-alanine and pantothenate. HPLC was used to assay the enzymatic activity of H. pylori 6x-his tagged ADC in the presence of different MA concentrations. H. pylori strains were serially exposed to MA for 14 passages, and the MICs were determined. Cytotoxicity in different cell lines was tested. The efficiency of ADC inhibition in treating H. pylori infections was evaluated using a Sprague-Dawley (SD) rat infection model. MA spectrum of activity was determined in different pathogens. MA binds to H. pylori ADC active site with a good docking score. The MIC of MA against H. pylori ranged from 0.5 to 0.75 mg/mL with MBC of 1.5 mg/mL. Increasing β-alanine and pantothenate concentrations proportionally increased MA MIC. The 6x-his tagged ADC activity decreased by increasing MA concentration. No resistance to ADC inhibition was recorded after 14 passages; MA lacked cytotoxicity in all tested cell lines. ADC inhibition effectively eradicated H. pylori infection in SD rats. MA had MIC between 0.625 to 1.25 mg/mL against the tested bacterial pathogens. In conclusion, ADC is a promising target for effectively eradicating H. pylori infection that is not affected by resistance development, besides being of broad-spectrum presence in different pathogens. MA provides a lead molecule for the development of an anti-helicobacter ADC inhibitor. This provides hope for saving the lives of those at high risk of infection with the carcinogenic H. pylori.
Keywords: Helicobacterpylori; aspartate α-decarboxylase; broad-spectrum; drug target; malonic acid.
Copyright © 2022 Ibrahim, Kashef, Elkhamissy, Ramadan and Helmy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Andersen L. P., Wadström T. (2001). “Basic bacteriology and culture,” in Helicobacter pylori: Physiology and genetics.. eds. Mobley H. L. T., Mendz G. L., Hazell S. L. (Washington, DC: ASM press; ), 132–154.
-
- Asgari B., Kermanian F., Yaghoobi M. H., Vaezi A., Soleimanifar F., Yaslianifard S. J. V. M. (2020). The anti-helicobacter pylori effects of lactobacillus acidophilus, L. plantarum, and L. rhamnosus in stomach tissue of C57BL/6 mice. Visceral Medecine 36, 137–143. doi: 10.1159/000500616, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

