JCS/JHRS 2020 Guideline on Pharmacotherapy of Cardiac Arrhythmias
- PMID: 36524037
- PMCID: PMC9745564
- DOI: 10.1002/joa3.12714
JCS/JHRS 2020 Guideline on Pharmacotherapy of Cardiac Arrhythmias
Figures
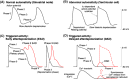
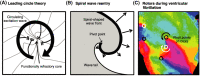



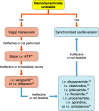
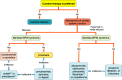




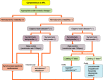


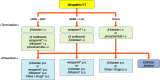
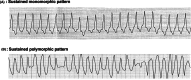
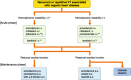



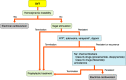

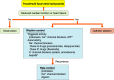
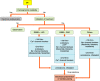
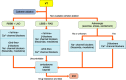
References
-
- Japanese Circulation Society Joint Working Group . Guidelines for Drug Treatment of Arrhythmias (JCS) 2009. [in Japanese] Available at: https://www.j‐circ.or.jp/cms/wp‐content/uploads/2020/02/JCS2009_kodama_h... (accessed October 6, 2021).
-
- Japanese Circulation Society Joint Working Group . Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2013). Circ J. 2014;78:1997–2021. - PubMed
-
- Mind Treatment Guideline Selection Committee . Fukui T, Yoshida M, Yamaguchi N, editors. Minds handbook for clinical practice guideline development. [in Japanese] Igaku‐Shoin, 2007.
-
- Japanese Circulation Society and Japanese Heart Rhythm Society Joint Working Group . JCS/JHRS 2019 guideline on non‐pharmacotherapy of cardiac arrhythmias. Circ J. 2021;85:1104–244. - PubMed
-
- Japanese Circulation Society Joint Working Group . Guidelines for Diagnosis and Management of Inherited Arrhythmias (JCS) 2017. [in Japanese] Available at: https://www.j‐circ.or.jp/cms/wp‐content/uploads/2020/02/JCS2017_aonuma_h... (accessed October 6, 2021).
LinkOut - more resources
Full Text Sources

