Identification and clinical characteristics of a novel missense ADGRG1 variant in bilateral Frontoparietal Polymicrogyria: The electroclinical change from infancy to adulthood after Callosotomy in three siblings
- PMID: 36524291
- PMCID: PMC9977754
- DOI: 10.1002/epi4.12685
Identification and clinical characteristics of a novel missense ADGRG1 variant in bilateral Frontoparietal Polymicrogyria: The electroclinical change from infancy to adulthood after Callosotomy in three siblings
Abstract
Objective: Bilateral frontoparietal polymicrogyria (BFPP) is a rare genetic-related migration disorder. It has been attributed to loss-of-function of the ADGRG1 gene, which encodes an adhesion G protein-coupled receptor, ADGRG1/GPR56. We report the EEG findings of BFPP in three Asian patients, and confirmed that change in protein function was caused by the novel missense variant (p.Leu290Pro).
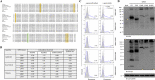
Methods: We reviewed the medical records of three siblings with BFPP including one elder girl and two identical twin boys from birth to adulthood. The clinical symptoms, electroencephalography (EEG), brain MRI, whole-exome sequencing, treatment including medications, neuromodulation, and epilepsy surgery, and clinical outcomes were reviewed. The protein structure of a novel missense variant (p.Leu290Pro) was predicted by in silico studies, and molecular analysis was performed via typical flow cytometry and Western blotting.
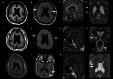
Results: The elder girl (Patient 1) was 22 years old and the twin boys (Patients 2 and 3) were 20 years old at the time of publication. All of them presented with typical clinical symptoms/signs and MRI findings of BFPP. Whole-exome sequencing followed by Sanger confirmation showed that all three patients had compound heterozygous variants in the ADGRG1 gene. The missense variant (p.Leu290Pro) was confirmed to be related to a reduction in cell surface GPR56 expression. High-amplitude rhythmic activity was noted in sleep EEG during infancy, which may have been due to excessive sleep spindle, and the rhythm disappeared when they were of pre-school age. Partial callosotomy provided short-term benefits in seizure control in Patients 1 and 2, and combined vagus nerve stimulation and partial callosotomy provided longer benefits in Patient 3.
Significance: Sleep EEG findings of high-amplitude rhythmic activity in our BFPP cases were only noted during infancy and childhood. We also confirmed that the missense variant (p.Leu290Pro) led to loss of function due to a reduction in cell surface GPR56 expression.
Keywords: congenital CNS malformation; epilepsy; genetic; polymicrogyria.
© 2022 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
None of the authors have any conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



References
-
- Piao X, Hill RS, Bodell A, Chang BS, Basel‐Vanagaite L, Straussberg R, et al. G protein‐coupled receptor‐dependent development of human frontal cortex. Science. 2004;303(5666):2033–6. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

