Recalibrating the calcium trap in amino acid carboxyl groups via classical molecular dynamics simulations
- PMID: 36524712
- PMCID: PMC9811642
- DOI: 10.1039/d2cp02879d
Recalibrating the calcium trap in amino acid carboxyl groups via classical molecular dynamics simulations
Abstract
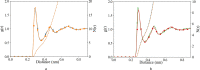
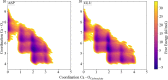
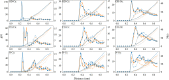
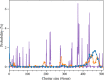
In order to use classical molecular dynamics to complement experiments accurately, it is important to use robust descriptions of the system. The interactions between biomolecules, like aspartic and glutamic acid, and dissolved ions are often studied using standard biomolecular force-fields, where the interactions between biomolecules and cations are often not parameterized explicitly. In this study, we have employed metadynamics simulations to investigate different interactions of Ca with aspartic and glutamic acid and constructed the free energy profiles of Ca2+-carboxylate association. Starting from a generally accepted, AMBER-based force field, the association was substantially over and under-estimated, depending on the choice of water model (TIP3P and SPC/fw, respectively). To rectify this discrepancy, we have replaced the default calcium parameters. Additionally, we modified the σij value in the hetero-atomic Lennard-Jones interaction by 0.5% to further improve the interaction between Ca and carboxylate, based on comparison with the experimentally determined association constant for Ca with the carboxylate group of L-aspartic acid. The corrected description retrieved the structural properties of the ion pair in agreement with the original biomolecule - Ca2+ interaction in AMBER, whilst also producing an association constant comparable to experimental observations. This refined force field was then used to investigate the interactions between amino acids, calcium and carbonate ions during biogenic and biomimetic calcium carbonate mineralisation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Force fields for divalent cations based on single-ion and ion-pair properties.J Chem Phys. 2013 Jan 14;138(2):024505. doi: 10.1063/1.4772808. J Chem Phys. 2013. PMID: 23320702
-
Simple physics-based analytical formulas for the potentials of mean force of the interaction of amino-acid side chains in water. V. Like-charged side chains.J Phys Chem B. 2011 May 19;115(19):6119-29. doi: 10.1021/jp111258p. Epub 2011 Apr 18. J Phys Chem B. 2011. PMID: 21500792 Free PMC article.
-
Interaction of charged amino-acid side chains with ions: an optimization strategy for classical force fields.J Phys Chem B. 2014 Apr 10;118(14):3960-72. doi: 10.1021/jp412490c. Epub 2014 Mar 31. J Phys Chem B. 2014. PMID: 24649981
-
Accounting for electronic polarization in non-polarizable force fields.Phys Chem Chem Phys. 2011 Feb 21;13(7):2613-26. doi: 10.1039/c0cp01971b. Epub 2011 Jan 7. Phys Chem Chem Phys. 2011. PMID: 21212894 Review.
-
Assessment of Simple Models for Molecular Simulation of Ethylene Carbonate and Propylene Carbonate as Solvents for Electrolyte Solutions.Top Curr Chem (Cham). 2018 Feb 12;376(2):7. doi: 10.1007/s41061-018-0187-2. Top Curr Chem (Cham). 2018. PMID: 29435669 Free PMC article. Review.
References
-
- Robin M. Von Euw S. Renaudin G. Gomes S. Krafft J. M. Nassif N. Azaïs T. Costentin G. Insights into OCP identification and quantification in the context of apatite biomineralization. CrystEngComm. 2020;22:2728–2742. doi: 10.1039/C9CE01972C. - DOI
-
- Hamm L., Calcium carbonate biomineralization: A theoretical and experimental investigation of biomolecular controls on nucleation and growth, 2012, pp. 1–130.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

