Origin of the Ligand Ring-Size Effect on the Catalytic Activity of Cationic Calcium Hydride Dimers in the Hydrogenation of Unactivated 1-Alkenes
- PMID: 36524742
- PMCID: PMC9756592
- DOI: 10.1002/open.202200240
Origin of the Ligand Ring-Size Effect on the Catalytic Activity of Cationic Calcium Hydride Dimers in the Hydrogenation of Unactivated 1-Alkenes
Abstract
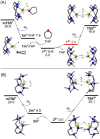
Recently, it was shown that the double Ca-H-Ca-bridged calcium hydride cation dimer [LCaH2 CaL]2+ when stabilized by a larger macrocyclic N,N',N'',N''',N''''-pentadentate ligand showed evidently higher activity than when stabilized by a smaller N,N',N'',N'''-tetradentate ligand in the catalytic hydrogenation of unactivated 1-alkenes. In this DFT-mechanistic work, the origin of the observed ring-size effect is examined in detail using 1-hexene, CH2 =CH2 and H2 as substrates. It is shown that, at room temperature, both the N,N',N'',N''',N''''-stabilized dimer and the monomer are not coordinated by THF in solution, while the corresponding N,N',N'',N'''-stabilized structures are coordinated by one THF molecule mimicking the fifth N-coordination. Catalytic 1-alkene hydrogenation may occur via anti-Markovnikov addition over the terminal Ca-H bonds of transient monomers, followed by faster Ca-C bond hydrogenolysis. The higher catalytic activity of the larger N,N',N'',N''',N''''-stabilized dimer is due to not only easier formation of but also due to the higher reactivity of the catalytic monomeric species. In contrast, despite unfavorable THF-coordination in solution, the smaller N,N',N'',N'''-stabilized dimer shows a 3.2 kcal mol-1 lower barrier via a dinuclear cooperative Ca-H-Ca bridge for H2 isotope exchange than the large N,N',N'',N''',N''''-stabilized dimer, mainly due to less steric hindrance. The observed ring-size effect can be understood mainly by a subtle interplay of solvent, steric and cooperative effects that can be resolved in detail by state-of-the-art quantum chemistry calculations.
Keywords: 1-alkene; calcium hydride complexes; homogenous catalysis; hydrogenation; isotope exchange.
© 2022 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Hill M. S., Liptrot D. J., Weetman C., Chem. Soc. Rev. 2016, 45, 972–988; - PubMed
-
- Harder S., Chem. Rev. 2010, 110, 3852–3876; - PubMed
-
- Spielmann J., Buch F., Harder S., Angew. Chem. Int. Ed. 2008, 47, 9434–9438; - PubMed
- Angew. Chem. 2008, 120, 9576–9580;
-
- Spielmann J., Harder S., Chem. Eur. J. 2007, 13, 8928–8938. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

