Plasma sphingolipid abnormalities in neurodegenerative diseases
- PMID: 36525454
- PMCID: PMC9757566
- DOI: 10.1371/journal.pone.0279315
Plasma sphingolipid abnormalities in neurodegenerative diseases
Abstract
Background: In recent years, there has been increasing evidence that several lipid metabolism abnormalities play an important role in the pathogenesis of neurodegenerative diseases. However, it is still unclear which lipid metabolism abnormalities play the most important role in neurodegenerative diseases. Plasma lipid metabolomics (lipidomics) has been shown to be an unbiased method that can be used to explore lipid metabolism abnormalities in neurodegenerative diseases. Plasma lipidomics in neurodegenerative diseases has been performed only in idiopathic Parkinson's disease (IPD) and Alzheimer's disease (AD), and comprehensive studies are needed to clarify the pathogenesis.
Methods: In this study, we investigated plasma lipids using lipidomics in individuals with neurodegenerative diseases and healthy controls (CNs). Plasma lipidomics was evaluated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in those with IPD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), AD, and progressive supranuclear palsy (PSP) and CNs.
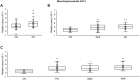
Results: The results showed that (1) plasma sphingosine-1-phosphate (S1P) was significantly lower in all neurodegenerative disease groups (IPD, DLB, MSA, AD, and PSP) than in the CN group. (2) Plasma monohexylceramide (MonCer) and lactosylceramide (LacCer) were significantly higher in all neurodegenerative disease groups (IPD, DLB, MSA, AD, and PSP) than in the CN group. (3) Plasma MonCer levels were significantly positively correlated with plasma LacCer levels in all enrolled groups.
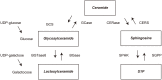
Conclusion: S1P, Glucosylceramide (GlcCer), the main component of MonCer, and LacCer are sphingolipids that are biosynthesized from ceramide. Recent studies have suggested that elevated GlcCer and decreased S1P levels in neurons are related to neuronal cell death and that elevated LacCer levels induce neurodegeneration by neuroinflammation. In the present study, we found decreased plasma S1P levels and elevated plasma MonCer and LacCer levels in those with neurodegenerative diseases, which is a new finding indicating the importance of abnormal sphingolipid metabolism in neurodegeneration.
Copyright: © 2022 Oizumi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Hogan DB, Fiest KM, Roberts JI, Maxwell CJ, Dykeman J, Pringsheim T, et al.. The prevalence and incidence of dementia with lewy bodies: a systematic review. Can J Neurol Sci. 2016;43 Suppl 1: S83–S95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

