β-Endorphin mediates radiation therapy fatigue
- PMID: 36525492
- PMCID: PMC9757747
- DOI: 10.1126/sciadv.abn6025
β-Endorphin mediates radiation therapy fatigue
Abstract
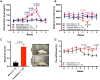
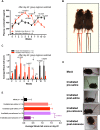
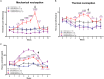
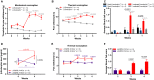
Fatigue is a common adverse effect of external beam radiation therapy in cancer patients. Mechanisms causing radiation fatigue remain unclear, although linkage to skin irradiation has been suggested. β-Endorphin, an endogenous opioid, is synthesized in skin following genotoxic ultraviolet irradiation and acts systemically, producing addiction. Exogenous opiates with the same receptor activity as β-endorphin can cause fatigue. Using rodent models of radiation therapy, exposing tails and sparing vital organs, we tested whether skin-derived β-endorphin contributes to radiation-induced fatigue. Over a 6-week radiation regimen, plasma β-endorphin increased in rats, paralleled by opiate phenotypes (elevated pain thresholds, Straub tail) and fatigue-like behavior, which was reversed in animals treated by the opiate antagonist naloxone. Mechanistically, all these phenotypes were blocked by opiate antagonist treatment and were undetected in either β-endorphin knockout mice or mice lacking keratinocyte p53 expression. These findings implicate skin-derived β-endorphin in systemic effects of radiation therapy. Opioid antagonism may warrant testing in humans as treatment or prevention of radiation-induced fatigue.
Figures
References
-
- Stasi R., Abriani L., Beccaglia P., Terzoli E., Amadori S.,Cancer-related fatigue. Cancer 98,1786–1801 (2003). - PubMed
-
- Greenberg D. B., Sawicka J., Eisenthal S., Ross D.,Fatigue syndrome due to localized radiation. J. Pain Symptom Manage. 7,38–45 (1992). - PubMed
-
- Jacobsen P. B., Thors C. L.,Fatigue in the radiation therapy patient: Current management and investigations. Semin. Radiat. Oncol. 13,372–380 (2003). - PubMed
-
- Hickok J. T., Morrow G. R., Roscoe J. A., Mustian K., Okunieff P.,Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J. Pain Symptom Manage. 30,433–442 (2005). - PubMed
-
- Back M., Ahern V., Delaney G., Graham P., Steigler A., Wratten C.; New South Wales Breast Radiation Oncology Group ,Absence of adverse early quality of life outcomes of radiation therapy in breast conservation therapy for early breast cancer. Australas. Radiol. 49,39–43 (2005). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

