EGFR is a master switch between immunosuppressive and immunoactive tumor microenvironment in inflammatory breast cancer
- PMID: 36525493
- PMCID: PMC9757751
- DOI: 10.1126/sciadv.abn7983
EGFR is a master switch between immunosuppressive and immunoactive tumor microenvironment in inflammatory breast cancer
Abstract
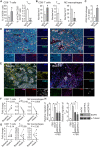
Inflammatory breast cancer (IBC), the most aggressive breast cancer subtype, is driven by an immunosuppressive tumor microenvironment (TME). Current treatments for IBC have limited efficacy. In a clinical trial (NCT01036087), an anti-EGFR antibody combined with neoadjuvant chemotherapy produced the highest pathological complete response rate ever reported in patients with IBC having triple-negative receptor status. We determined the molecular and immunological mechanisms behind this superior clinical outcome. Using novel humanized IBC mouse models, we discovered that EGFR-targeted therapy remodels the IBC TME by increasing cytotoxic T cells and reducing immunosuppressive regulatory T cells and M2 macrophages. These changes were due to diminishing immunosuppressive chemokine expression regulated by transcription factor EGR1. We also showed that induction of an immunoactive IBC TME by an anti-EGFR antibody improved the antitumor efficacy of an anti-PD-L1 antibody. Our findings lay the foundation for clinical trials evaluating EGFR-targeted therapy combined with immune checkpoint inhibitors in patients with cancer.
Figures




References
-
- P. Schmid, J. Cortes, L. Pusztai, H. McArthur, S. Kummel, J. Bergh, C. Denkert, Y. H. Park, R. Hui, N. Harbeck, M. Takahashi, T. Foukakis, P. A. Fasching, F. Cardoso, M. Untch, L. Jia, V. Karantza, J. Zhao, G. Aktan, R. Dent, J. O’Shaughnessy; KEYNOTE-522 Investigators ,Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020). - PubMed
-
- E. A. Mittendorf, H. Zhang, C. H. Barrios, S. Saji, K. H. Jung, R. Hegg, A. Koehler, J. Sohn, H. Iwata, M. L. Telli, C. Ferrario, K. Punie, F. Penault-Llorca, S. Patel, A. N. Duc, M. Liste-Hermoso, V. Maiya, L. Molinero, S. Y. Chui, N. Harbeck,Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100 (2020). - PubMed
-
- L. Gandhi, D. Rodriguez-Abreu, S. Gadgeel, E. Esteban, E. Felip, F. De Angelis, M. Domine, P. Clingan, M. J. Hochmair, S. F. Powell, S. Y. Cheng, H. G. Bischoff, N. Peled, F. Grossi, R. R. Jennens, M. Reck, R. Hui, E. B. Garon, M. Boyer, B. Rubio-Viqueira, S. Novello, T. Kurata, J. E. Gray, J. Vida, Z. Wei, J. Yang, H. Raftopoulos, M. C. Pietanza, M. C. Garassino; KEYNOTE-189 Investigators ,Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018). - PubMed
-
- L. Paz-Ares, A. Luft, D. Vicente, A. Tafreshi, M. Gumus, J. Mazieres, B. Hermes, F. Cay Senler, T. Csoszi, A. Fulop, J. Rodriguez-Cid, J. Wilson, S. Sugawara, T. Kato, K. H. Lee, Y. Cheng, S. Novello, B. Halmos, X. Li, G. M. Lubiniecki, B. Piperdi, D. M. Kowalski; KEYNOTE-407 Investigators ,Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018). - PubMed
-
- C. Robert, G. V. Long, B. Brady, C. Dutriaux, M. Maio, L. Mortier, J. C. Hassel, P. Rutkowski, C. McNeil, E. Kalinka-Warzocha, K. J. Savage, M. M. Hernberg, C. Lebbe, J. Charles, C. Mihalcioiu, V. Chiarion-Sileni, C. Mauch, F. Cognetti, A. Arance, H. Schmidt, D. Schadendorf, H. Gogas, L. Lundgren-Eriksson, C. Horak, B. Sharkey, I. M. Waxman, V. Atkinson, P. A. Ascierto,Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

