In vivo characterization of glutamine metabolism identifies therapeutic targets in clear cell renal cell carcinoma
- PMID: 36525494
- PMCID: PMC9757752
- DOI: 10.1126/sciadv.abp8293
In vivo characterization of glutamine metabolism identifies therapeutic targets in clear cell renal cell carcinoma
Abstract
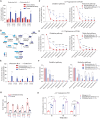
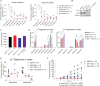
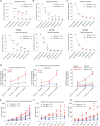
Targeting metabolic vulnerabilities has been proposed as a therapeutic strategy in renal cell carcinoma (RCC). Here, we analyzed the metabolism of patient-derived xenografts (tumorgrafts) from diverse subtypes of RCC. Tumorgrafts from VHL-mutant clear cell RCC (ccRCC) retained metabolic features of human ccRCC and engaged in oxidative and reductive glutamine metabolism. Genetic silencing of isocitrate dehydrogenase-1 or isocitrate dehydrogenase-2 impaired reductive labeling of tricarboxylic acid (TCA) cycle intermediates in vivo and suppressed growth of tumors generated from tumorgraft-derived cells. Glutaminase inhibition reduced the contribution of glutamine to the TCA cycle and resulted in modest suppression of tumorgraft growth. Infusions with [amide-15N]glutamine revealed persistent amidotransferase activity during glutaminase inhibition, and blocking these activities with the amidotransferase inhibitor JHU-083 also reduced tumor growth in both immunocompromised and immunocompetent mice. We conclude that ccRCC tumorgrafts catabolize glutamine via multiple pathways, perhaps explaining why it has been challenging to achieve therapeutic responses in patients by inhibiting glutaminase.
Figures


Comment in
-
Targeting glutamine use in RCC.Nat Rev Nephrol. 2023 Mar;19(3):151. doi: 10.1038/s41581-023-00684-2. Nat Rev Nephrol. 2023. PMID: 36694057 No abstract available.
References
-
- W. M. Linehan, L. S. Schmidt, D. R. Crooks, D. Wei, R. Srinivasan, M. Lang, C. J. Ricketts,The metabolic basis of kidney cancer. Cancer Discov. 9,1006–1021 (2019). - PubMed
-
- X. Ma, K. Yang, P. Lindblad, L. Egevad, K. Hemminki,VHL gene alterations in renal cell carcinoma patients: Novel hotspot or founder mutations and linkage disequilibrium. Oncogene 20,5393–5400 (2001). - PubMed
-
- D. Tarade, M. Ohh,The HIF and other quandaries in VHL disease. Oncogene 37,139–147 (2018). - PubMed
-
- G. L. Semenza, B. H. Jiang, S. W. Leung, R. Passantino, J. P. Concordet, P. Maire, A. Giallongo,Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 271,32529–32537 (1996). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

