Efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections associated with low procalcitonin: a randomised, placebo-controlled, double-blind, non-inferiority trial
- PMID: 36525985
- PMCID: PMC10040424
- DOI: 10.1016/S1473-3099(22)00735-6
Efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections associated with low procalcitonin: a randomised, placebo-controlled, double-blind, non-inferiority trial
Abstract
Background: Lower respiratory tract infections are frequently treated with antibiotics, despite a viral cause in many cases. It remains unknown whether low procalcitonin concentrations can identify patients with lower respiratory tract infection who are unlikely to benefit from antibiotics. We aimed to compare the efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections in patients with low procalcitonin.
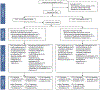
Methods: We conducted a randomised, placebo-controlled, double-blind, non-inferiority trial at five health centres in the USA. Adults aged 18 years or older with clinically suspected non-pneumonia lower respiratory tract infection and symptom duration from 24 h to 28 days were eligible for enrolment. Participants with a procalcitonin concentration of 0·25 ng/mL or less were randomly assigned (1:1), in blocks of four with stratification by site, to receive over-encapsulated oral azithromycin 250 mg or matching placebo (two capsules on day 1 followed by one capsule daily for 4 days). Participants, non-study clinical providers, investigators, and study coordinators were masked to treatment allocation. The primary outcome was efficacy of azithromycin versus placebo in terms of clinical improvement at day 5 in the intention-to-treat population. The non-inferiority margin was -12·5%. Solicited adverse events (abdominal pain, vomiting, diarrhoea, allergic reaction, or yeast infections) were recorded as a secondary outcome. This trial is registered with ClinicalTrials.gov, NCT03341273.
Findings: Between Dec 8, 2017, and March 9, 2020, 691 patients were assessed for eligibility and 499 were enrolled and randomly assigned to receive azithromycin (n=249) or placebo (n=250). Clinical improvement at day 5 was observed in 148 (63%, 95% CI 54 to 71) of 238 participants with full data in the placebo group and 155 (69%, 61 to 77) of 227 participants with full data in the azithromycin group in the intention-to-treat analysis (between-group difference -6%, 95% CI -15 to 2). The 95% CI for the difference did not meet the non-inferiority margin. Solicited adverse events and the severity of solicited adverse events were not significantly different between groups at day 5, except for increased abdominal pain associated with azithromycin (47 [23%, 95% CI 18 to 29] of 204 participants) compared with placebo (35 [16%, 12 to 21] of 221; between-group difference -7% [95% CI -15 to 0]; p=0·066).
Interpretation: Placebo was not non-inferior to azithromycin in terms of clinical improvement at day 5 in adults with lower respiratory tract infection and a low procalcitonin concentration. After accounting for both the rates of clinical improvement and solicited adverse events at day 5, it is unclear whether antibiotics are indicated for patients with lower respiratory tract infection and a low procalcitonin concentration.
Funding: National Institute of Allergy and Infectious Diseases, bioMérieux.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests ELT and CWW report consulting for Biomeme. ELT, CWW, and MTM have a patent pending for Methods to Diagnose and Treat Acute Respiratory Infections (US20180245154A1). Following completion of the study, ELT became an employee of Danaher Diagnostics. EBW is a principal investigator for Pfizer-funded studies of COVID-19 vaccine, a co-investigator for a vaccine study funded by Moderna, and a member of an advisory board for Vaxcyte. NGR serves as a safety consultant for the Emmes Corporation and ICON-I, and reports research funds from Pfizer, Merck, Sanofi, Quidel, and Lilly. All other authors declare no competing interests.
Figures


Comment in
-
Procalcitonin to reduce antimicrobial overuse in patients with lower respiratory tract infection: time for re-evaluation of our prescription culture?Lancet Infect Dis. 2023 Apr;23(4):390-391. doi: 10.1016/S1473-3099(22)00757-5. Epub 2022 Dec 13. Lancet Infect Dis. 2023. PMID: 36525986 No abstract available.
-
Seasonal variation in azithromycin prescription.Lancet Infect Dis. 2023 Mar;23(3):277-278. doi: 10.1016/S1473-3099(23)00009-9. Epub 2023 Jan 31. Lancet Infect Dis. 2023. PMID: 36736337 No abstract available.
References
-
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr., et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA. 2016;315(17): 1864–73. - PubMed
-
- Kamat IS, Ramachandran V, Eswaran H, Guffey D, Musher DM. Procalcitonin to Distinguish Viral From Bacterial Pneumonia: A Systematic Review and Meta-analysis. Clin Infect Dis. 2020;70(3):538–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

