Mechanosensitive ion channel Piezo1 mediates mechanical ventilation-exacerbated ARDS-associated pulmonary fibrosis
- PMID: 36526145
- PMCID: PMC10658225
- DOI: 10.1016/j.jare.2022.12.006
Mechanosensitive ion channel Piezo1 mediates mechanical ventilation-exacerbated ARDS-associated pulmonary fibrosis
Abstract
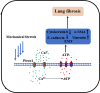
Introduction: Pulmonary fibrosis is a major cause of the poor prognosis of acute respiratory distress syndrome (ARDS). While mechanical ventilation (MV) is an indispensable life-saving intervention for ARDS, it may cause the remodeling process in lung epithelial cells to become disorganized and exacerbate ARDS-associated pulmonary fibrosis. Piezo1 is a mechanosensitive ion channel that is known to play a role in regulating diverse physiological processes, but whether Piezo1 is necessary for MV-exacerbated ARDS-associated pulmonary fibrosis remains unknown.
Objectives: This study aimed to explore the role of Piezo1 in MV-exacerbated ARDS-associated pulmonary fibrosis.
Methods: Human lung epithelial cells were stimulated with hydrochloric acid (HCl) followed by mechanical stretch for 48 h. A two-hitmodel of MV afteracidaspiration-inducedlunginjuryin mice was used. Mice were sacrificed after 14 days of MV. Pharmacological inhibition and knockout of Piezo1 were used to delineate the role of Piezo1 in MV-exacerbated ARDS-associated pulmonary fibrosis. In some experiments, ATP or the ATP-hydrolyzing enzyme apyrase was administered.
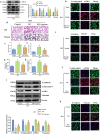
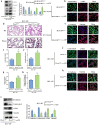
Results: The stimulation of human lung epithelial cells to HCl resulted in phenotypes of epithelial-mesenchymal transition (EMT), which were enhanced by mechanical stretching. MV exacerbated pulmonary fibrosis in mice exposed to HCl. Pharmacologicalinhibitionorknockout of Piezo1 attenuated the MV-exacerbated EMT process and lung fibrosis in vivo and in vitro. Mechanistically, the observed effects were mediated by Piezo1-dependent Ca2+ influx and ATP release in lung epithelial cells.
Conclusions: Our findings identify a key role for Piezo1 in MV-exacerbated ARDS-associated pulmonary fibrosis that is mediated by increased ATP release in lung epithelial cells. Inhibiting Piezo1 may constitute a novelstrategyfor the treatment of MV-exacerbated ARDS-associated pulmonary fibrosis.
Keywords: Acute respiratory distress syndrome; Mechanical ventilation; Piezo1; Pulmonary fibrosis.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Tomashefski J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. - PubMed
-
- Rocco P.R., Dos Santos C., Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75:730–740. - PubMed
-
- Thille A.W., Esteban A., Fernández-Segoviano P., Rodriguez J.M., Aramburu J.A., Vargas-Errázuriz P., et al. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

