Outcomes in Patients with FLT3-Mutated Relapsed/ Refractory Acute Myelogenous Leukemia Who Underwent Transplantation in the Phase 3 ADMIRAL Trial of Gilteritinib versus Salvage Chemotherapy
- PMID: 36526260
- PMCID: PMC10189888
- DOI: 10.1016/j.jtct.2022.12.006
Outcomes in Patients with FLT3-Mutated Relapsed/ Refractory Acute Myelogenous Leukemia Who Underwent Transplantation in the Phase 3 ADMIRAL Trial of Gilteritinib versus Salvage Chemotherapy
Abstract
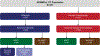
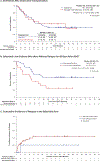
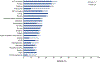
The fms-like tyrosine kinase 3 (FLT3) inhibitor gilteritinib improved the survival of patients with relapsed or refractory (R/R) FLT3-mutated acute myelogenous leukemia (AML) in the phase 3 ADMIRAL trial. In this study, we assessed survival and relapse rates of patients in the ADMIRAL trial who underwent hematopoietic stem cell transplantation (HSCT), as well as safety outcomes in patients who received post-transplantation gilteritinib maintenance therapy. ADMIRAL was a global phase 3 randomized controlled trial that enrolled adult patients with FLT3-mutated R/R AML. Patients with R/R AML who harbored FLT3 internal tandem duplication mutations in the juxtamembrane domain or D835/I836 point mutations in the tyrosine kinase domain were randomized (2:1) to gilteritinib (120 mg/day) or to preselected high- or low-intensity salvage chemotherapy (1 or 2 cycles). Patients in the gilteritinib arm who proceeded to HSCT could receive post-transplantation gilteritinib maintenance therapy if they were within 30 to 90 days post-transplantation and had achieved composite complete remission (CRc) with successful engraftment and no post-transplantation complications. Adverse events (AEs) during HSCT were recorded in the gilteritinib arm only. Survival outcomes and the cumulative incidence of relapse were assessed in patients who underwent HSCT during the trial. Treatment-emergent AEs were evaluated in patients who restarted gilteritinib as post-transplantation maintenance therapy. Patients in the gilteritinib arm underwent HSCT more frequently than those in the chemotherapy arm (26% [n = 64] versus 15% [n = 19]). For all transplantation recipients, 12- and 24-month overall survival (OS) rates were 68% and 47%, respectively. Despite a trend toward longer OS after pretransplantation CRc, post-transplantation survival was comparable in the 2 arms. Patients who resumed gilteritinib after HSCT had a low relapse rate after pretransplantation CRc (20%) or CR (0%). The most common AEs observed with post-transplantation gilteritinib therapy were increased alanine aminotransferase level (45%), pyrexia (43%), and diarrhea (40%); grade ≥3 AEs were related primarily to myelosuppression. The incidences of grade ≥III acute graft-versus-host disease and related mortality were low. Post-transplantation survival was similar across the 2 study arms in the ADMIRAL trial, but higher remission rates with gilteritinib facilitated receipt of HSCT. Gilteritinib as post-transplantation maintenance therapy had a stable safety and tolerability profile and was associated with low relapse rates. Taken together, these data support a preference for bridging therapy with gilteritinib over chemotherapy in transplantation-eligible patients.
Keywords: Acute myelogenous leukemia; FLT3 inhibitor; FLT3 mutation; Hematopoietic stem cell transplantation; Post-transplantation maintenance therapy.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
A.E.P. reports funding and other support from Astellas for this study; grants and contracts from AbbVie, Actinum Pharmaceuticals, Astellas, Daiichi Sankyo, Fujifilm, and Bayer; consulting fees from AbbVie, Astellas, Daiichi Sankyo, Forma Therapeutics, Sumitomo Dainippon Pharma, and Onconova; honoraria from Astellas and Daiichi Sankyo; and other financial support related to conference attendance and serving on advisory or data safety monitoring boards sponsored by Daiichi Sankyo, AbbVie, Astellas, Actinium, Celgene/Bristol-Myers Squibb, Genentech, Loxo, Syndax, Agios, Takeda, NewLink Genetics, and the Leukemia & Lymphoma Society/Beat AML, all outside the submitted work. R.A.L. reports funding and other support from Astellas for this study; grants or contracts from Cellectis, Forty Seven/Gilead Sciences, Rafael Pharmaceuticals, Novartis, and Daiichi Sankyo; consulting fees from Novartis, Agios, and CVS/Caremark; royalties from UpToDate; other financial support from Amgen and Novartis for conference attendance; and financial support for serving on data safety monitoring boards sponsored by Ariad/Takeda and Celgene/Bristol-Myers Squibb and on an external data review committee for Epizyme, all outside the submitted work. N.A.P. reports funding from Astellas for this study; consulting fees from Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene/Bristol-Myers Squibb, CTI BioPharma, PharmaEssentia, Constellation Pharmaceuticals, and AbbVie; and other financial support for serving on an independent data review committee for Cogent Biosciences, all outside the submitted work. S.S. reports other financial support for serving on advisory boards sponsored by AbbVie, Ber-GenBio, Astellas, Genentech, Kura Oncology, Novartis, Senti Bio, and Syros, all outside the submitted work. E.S.W. reports funding from Astellas for this study; consulting fees from Mana Therapeutics; honoraria from Stemline Therapeutics, Kura Oncology, Pfizer, and Dava Oncology; other financial support for serving on advisory boards sponsored by AbbVie, Astellas, Celgene/Bristol-Myers Squibb, Genentech, Gilead Sciences, GlaxoSmithKline, Jazz, Kite, Kura Oncology, Novartis, Pfizer, Stemline Therapeutics, and Takeda; and financial support for serving on data monitoring committees for AbbVie and Rafael Pharmaceuticals, all outside the submitted work. E.A. reports grants or contracts from Novartis and Takeda; other financial support for serving on advisory boards sponsored by Novartis, Takeda, Syndax, Bristol-Myers Squibb, AbbVie, Adaptive Biotechnologies, and Amgen; and other financial support for serving on advisory boards sponsored by AbbVie, Amgen, and Syndax and a data safety monitoring board sponsored by Takeda; and medical writing support from Novartis, all outside the submitted work. G.J.S. reports funding from Astellas for this study and honoraria from Astellas outside the submitted work. J.S. reports funding from Astellas for this study and honoraria for participation in a speaker program sponsored by Astellas, grant funding from Novartis, as well as personal fees from Astellas, Jazz, AbbVie, Daiichi Sankyo, Pfizer, and Novartis, all outside the submitted work. G.M. reports funding from Astellas for this study. P.M. reports funding from Astellas for this study and for the development of this manuscript and other grant funding from Astellas outside the submitted work. C.R. reports grants or contracts from AbbVie, Amgen, Novartis, Celgene/Bristol-Myers Squibb, Jazz, Agios, Daiichi Sankyo, Astellas, Sunesis, Roche, and MaatPharma; consulting fees from AbbVie, Jazz, Novartis, and Celgene/Bristol Myers-Squibb; honoraria from Astellas; and other financial support from Novartis, Gilead, Sanofi, Jazz, Amgen, and Daiichi Sankyo for travel/conference attendance, all outside the submitted work. S.S.Y. reports research grants from Roche-Genentech, Kyowa Kirin, and Yuhan Pharmaceuticals, as well as funding for participation on advisory boards sponsored by Amgen, Astellas, Celgene, Janssen, Novartis, and Takeda, all outside the submitted work. Y.M. reports funding from Astellas for this study and research funding from Astellas outside the submitted work. T.K. reports funding from Astellas for this study. H.J.K. reports honoraria and consultancy fees from Amgen, Astellas, Celgene/Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Janssen, SL VaxiGen, and Yuhan Pharma; honoraria from AbbVie; and research funding from BL&H Co Ltd., all outside the submitted work. M.J.L. reports funding from Astellas for this study; research grants from Astellas and FujiFilm; and honoraria from Amgen, AbbVie, Astellas, Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Menarini, and Takeda, all outside the submitted work. N.H. and R.N. are employees of Astellas. R.T. is a former employee of Astellas. A.N., M.O., and N.H. have no conflicts of interest to report.
Figures


References
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. - PubMed
-
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. - PubMed
-
- Bazarbachi A, Bug G, Baron F, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2020;105:1507–1516. - PMC - PubMed
-
- Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters–an analysis of 3082 patients. Blood. 2008; 111:2527–2537. - PubMed

