Significance of Myelin Oligodendrocyte Glycoprotein Antibodies in CSF: A Retrospective Multicenter Study
- PMID: 36526426
- PMCID: PMC10074465
- DOI: 10.1212/WNL.0000000000201662
Significance of Myelin Oligodendrocyte Glycoprotein Antibodies in CSF: A Retrospective Multicenter Study
Abstract
Background and objectives: Although the diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is based on serum MOG antibodies (MOG-Abs) positivity, patients with coexisting or restricted MOG-Abs in the CSF have been reported. The aim of this study is to characterize the relevance of CSF MOG-Abs positivity in clinical practice.
Methods: Eleven medical centers retrospectively collected clinical and laboratory data of adult and pediatric patients with suspected inflammatory CNS disease and MOG-Abs positivity in serum and/or CSF using live cell-based assays. Comparisons were performed using parametric or nonparametric tests, as appropriate. Potential factors of unfavorable outcomes were explored by Cox proportional hazard models and logistic regression.
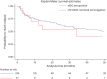
Results: The cohort included 255 patients: 139 (55%) women and 132 (52%) children (i.e., <18-year-old). Among them, 145 patients (56.8%) had MOG-Abs in both serum and CSF (MOG-Abs seropositive and CSF positive), 79 (31%) only in serum (MOG-Abs seropositive and CSF negative), and 31 (12%) only in CSF (MOG-Abs seronegative and CSF positive). MOG-Abs seronegative and CSF positive predominated in adults (22% vs 3% of children), presented more commonly with motor (n = 14, 45%) and sensory symptoms (n = 13, 42%), and all but 4 (2 multiple sclerosis, 1 polyradiculoneuritis, and 1 Susac syndrome) had a final diagnosis compatible with MOGAD. When comparing seropositive patients according to MOG-Abs CSF status, MOG-Abs seropositive and CSF positive patients had a higher Expanded Disability Status Scale (EDSS) at nadir during the index event (median 4.5, interquartile range [IQR] 3.0-7.5 vs 3.0, IQR 2.0-6.8, p = 0.007) and presented more commonly with sensory (45.5% vs 24%, p = 0.002), motor (33.6% vs 19%, p = 0.021), and sphincter symptoms (26.9% vs 7.8%, p = 0.001) than MOG-Abs seropositive and CSF negative. At the last follow-up, MOG-Abs seropositive and CSF positive cases had more often persistent sphincter dysfunction (17.3% vs 4.3%, p = 0.008). Compared with seropositive patients, those MOG-Abs seronegative and CSF positive had higher disability at the last follow-up (p ≤ 0.001), and MOG-Abs seronegative and CSF positive status were independently associated with an EDSS ≥3.0.
Discussion: Paired serum and CSF MOG-Abs positivity are common in MOGAD and are associated with a more severe clinical presentation. CSF-only MOG-Abs positivity can occur in patients with a phenotype suggestive of MOGAD and is associated with a worse outcome. Taken together, these data suggest a clinical interest in assessing CSF MOG-Abs in patients with a phenotype suggestive of MOGAD, regardless of the MOG-Abs serostatus.
© 2022 American Academy of Neurology.
Conflict of interest statement
T. Armangué received speaker honoraria from Biogen, Sanofi-Aventis, and Novartis not related to this manuscript; A. Saiz reports compensation for consulting services and speaker honoraria from Merck-Serono, Biogen-Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, Novartis, Roche, Alexion, and Janssen; C. Lechner has served as a consultant for Roche but has no conflict of interest with this manuscript; I. Ayzenberg served on scientific advisory boards for Roche, Merck, and Alexion and received research support from Diamed, none related to this article; M. Sepulveda has received speaker honoraria from Biogen and Roche Pharma; E. Martínez-Hernández has received speaker honoraria from Biogen; G. Arrambide has received speaking honoraria and compensation for consulting services or participation in advisory boards from Sanofi, Merck, and Roche; travel expenses for scientific meetings from Novartis, Roche, Stendhal, and ECTRIMS; is editor for Europe of the Multiple Sclerosis Journal – Experimental, Translational, and Clinical; and is a member of the International Women in Multiple Sclerosis (iWiMS) network executive committee. F. Brilot has received research funding from the National Health and Medical Research Council (Australia), Multiple Sclerosis Research Australia, NSW Health, Novartis, and the University of Sydney. She has received speaker honoraria from Novartis, Biogen, Merck, and Limbic Neurology, has been on advisory boards for Merck and Novartis. and is a member of the International Women in Multiple Sclerosis (iWiMS) network executive committee. S. Ramanathan has received research funding from the National Health and Medical Research Council (Australia), the Brain Foundation (Australia), the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Neil Hamilton Fairley Early Career Fellowship (APP1141169). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for Biogen, EXCEMED, and Limbic Neurology. S. Ferrari received support for attending scientific meetings by Shire, Sanofi-Genzyme, Roche, and Euroimmun; E. P. Flanagan has served on advisory boards for Alexion, Genentech, and Horizon Therapeutics. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. He was a site primary investigator in a randomized clinical trial on inebilizumab in neuromyelitis optica spectrum disorder run by MedImmune/Viela-Bio/Horizon Therapeutics. He has received funding from the NIH (R01NS113828). He is a member of the medical advisory board of the MOG project. He is an editorial board member of the Journal of the Neurologic Sciences and Neuroimmunology Reports. A patent has been submitted on DACH1-IgG as a biomarker of paraneoplastic autoimmunity. M. Reindl is supported by research grants from the Austrian Science Fund (FWF project P32699), the Austrian Research Promotion Agency, Euroimmun, and Roche; consulting fees and advisory board from Roche (to institution) and payments for antibody assays (MOG, AQP4, and other autoantibodies) to institution (University Hospital and Medical University of Innsbruck, Austria). R. Marignier serves on scientific advisory board for Alexion, Horizon Therapeutics, Roche, and UCB; has received honoraria from Alexion, Biogen, Merck, Novartis, and Roche. S. Mariotto received support for attending scientific meetings by Merck and Euroimmun and received speaker honoraria from Biogen. The other authors report no relevant disclosures. Go to
Figures

Comment in
-
Should We test for IgG Antibodies Against MOG in Both Serum and CSF in Patients With Suspected MOGAD?Neurology. 2023 Mar 14;100(11):497-498. doi: 10.1212/WNL.0000000000206805. Epub 2022 Dec 16. Neurology. 2023. PMID: 36526427 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
