The promising approach of MSCs therapy for COVID-19 treatment
- PMID: 36526819
- PMCID: PMC9757632
- DOI: 10.1007/s10561-022-10060-2
The promising approach of MSCs therapy for COVID-19 treatment
Abstract
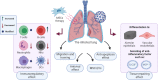
Several ongoing investigations have been founded on the development of an optimized therapeutic strategy for the COVID-19 virus as an undeniable acute challenge for human life. Cell-based therapy and particularly, mesenchymal stem cells (MSCs) therapy has obtained desired outcomes in decreasing the mortality rate of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), mainly owing to its immunoregulatory impact that prevents the overactivation of the immune system. Also, these cells with their multipotent nature, are capable of repairing the damaged tissue of the lung which leads to reducing the probability of acute respiratory distress syndrome (ARDS). Although this cell-based method is not quite cost-effective for developing countries, regarding its promising results in order to treat SARS-COV-2, more economical evaluation as well as clinical trials should be performed for improving this therapeutic approach. Here in this article, the functional mechanism of MSCs therapy for the treatment of COVID-19 and the clinical trials based on this method will be reviewed. Moreover, its economic efficiency will be discussed.
Keywords: Acute respiratory distress dyndrome (ARDS); COVID-19; Cell therapy; Clinical trials; Mesenchymal stem cells (MSCs).
© 2022. The Author(s), under exclusive licence to Springer Nature B.V.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous