Targeting USP10 induces degradation of oncogenic ANLN in esophageal squamous cell carcinoma
- PMID: 36526897
- PMCID: PMC9950447
- DOI: 10.1038/s41418-022-01104-x
Targeting USP10 induces degradation of oncogenic ANLN in esophageal squamous cell carcinoma
Abstract
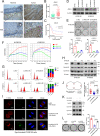
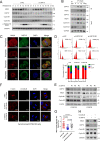
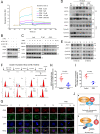
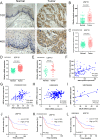
Anillin (ANLN) is a mitosis-related protein that promotes contractile ring formation and cytokinesis, but its cell cycle-dependent degradation mechanisms in cancer cells remain unclear. Here, we show that high expression of ANLN promotes cytokinesis and proliferation in esophageal squamous cell carcinoma (ESCC) cells and is associated with poor prognosis in ESCC patients. Furthermore, the findings of the study showed that the deubiquitinating enzyme USP10 interacts with ANLN and positively regulates ANLN protein levels. USP10 removes the K11- and K63-linked ubiquitin chains of ANLN through its deubiquitinase activity and prevents ANLN ubiquitin-mediated degradation. Importantly, USP10 promotes contractile ring assembly at the cytokinetic furrow as well as cytokinesis by stabilizing ANLN. Interestingly, USP10 and the E3 ubiquitin ligase APC/C co-activator Cdh1 formed a functional complex with ANLN in a non-competitive manner to balance ANLN protein levels. In addition, the macrolide compound FW-04-806 (F806), a natural compound with potential for treating ESCC, inhibited the mitosis of ESCC cells by targeting USP10 and promoting ANLN degradation. F806 selectively targeted USP10 and inhibited its catalytic activity but did not affect the binding of Cdh1 to ANLN and alters the balance of the USP10-Cdh1-ANLN complex. Additionally, USP10 expression was positively correlated with ANLN level and poor prognosis of ESCC patients. Overall, targeting the USP10-ANLN axis can effectively inhibit ESCC cell-cycle progression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

