Annexin A2 modulates phospholipid membrane composition upstream of Arp2 to control angiogenic sprout initiation
- PMID: 36527391
- PMCID: PMC10586062
- DOI: 10.1096/fj.202201088R
Annexin A2 modulates phospholipid membrane composition upstream of Arp2 to control angiogenic sprout initiation
Abstract
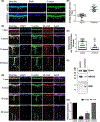
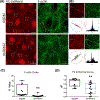
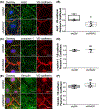
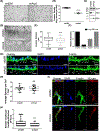
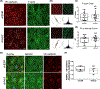
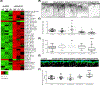
The intersection of protein and lipid biology is of growing importance for understanding how cells address structural challenges during adhesion and migration. While protein complexes engaged with the cytoskeleton play a vital role, support from the phospholipid membrane is crucial for directing localization and assembly of key protein complexes. During angiogenesis, dramatic cellular remodeling is necessary for endothelial cells to shift from a stable monolayer to invasive structures. However, the molecular dynamics between lipids and proteins during endothelial invasion are not defined. Here, we utilized cell culture, immunofluorescence, and lipidomic analyses to identify a novel role for the membrane binding protein Annexin A2 (ANXA2) in modulating the composition of specific membrane lipids necessary for cortical F-actin organization and adherens junction stabilization. In the absence of ANXA2, there is disorganized cortical F-actin, reduced junctional Arp2, excess sprout initiation, and ultimately failed sprout maturation. Furthermore, we observed reduced filipin III labeling of membrane cholesterol in cells with reduced ANXA2, suggesting there is an alteration in phospholipid membrane dynamics. Lipidomic analyses revealed that 42 lipid species were altered with loss of ANXA2, including an accumulation of phosphatidylcholine (16:0_16:0). We found that supplementation of phosphatidylcholine (16:0_16:0) in wild-type endothelial cells mimicked the ANXA2 knock-down phenotype, indicating that ANXA2 regulated the phospholipid membrane upstream of Arp2 recruitment and organization of cortical F-actin. Altogether, these data indicate a novel role for ANXA2 in coordinating events at endothelial junctions needed to initiate sprouting and show that proper lipid modulation is a critical component of these events.
Keywords: Annexin A2; actin-related protein 2; adherens junctions; cholesterol; cytoskeleton; endothelial cells; lipidomics; membrane lipids; phosphatidylcholines; phospholipids.
© 2022 Federation of American Societies for Experimental Biology.
Conflict of interest statement
DISCLOSURES
The authors have no competing interests in this work, financial or otherwise.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

