Food memory circuits regulate eating and energy balance
- PMID: 36528025
- PMCID: PMC9877168
- DOI: 10.1016/j.cub.2022.11.039
Food memory circuits regulate eating and energy balance
Abstract
In mammals, learning circuits play an essential role in energy balance by creating associations between sensory cues and the rewarding qualities of food. This process is altered by diet-induced obesity, but the causes and mechanisms are poorly understood. Here, we exploited the relative simplicity and wealth of knowledge about the D. melanogaster reinforcement learning network, the mushroom body, in order to study the relationship between the dietary environment, dopamine-induced plasticity, and food associations. We show flies that are fed a high-sugar diet cannot make associations between sensory cues and the rewarding properties of sugar. This deficit was caused by diet exposure, not fat accumulation, and specifically by lower dopamine-induced plasticity onto mushroom body output neurons (MBONs) during learning. Importantly, food memories dynamically tune the output of MBONs during eating, which instead remains fixed in sugar-diet animals. Interestingly, manipulating the activity of MBONs influenced eating and fat mass, depending on the diet. Altogether, this work advances our fundamental understanding of the mechanisms, causes, and consequences of the dietary environment on reinforcement learning and ingestive behavior.
Keywords: nutrition; reinforcement learning; sensory plasticity; taste.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

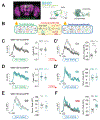
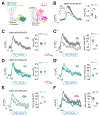



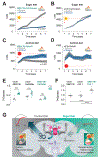
Comment in
-
Eating regulation: How diet impacts food cognition.Curr Biol. 2023 Feb 27;33(4):R153-R156. doi: 10.1016/j.cub.2022.12.060. Curr Biol. 2023. PMID: 36854275
Similar articles
-
Dietary sugar inhibits satiation by decreasing the central processing of sweet taste.Elife. 2020 Jun 16;9:e54530. doi: 10.7554/eLife.54530. Elife. 2020. PMID: 32539934 Free PMC article.
-
Reinforcement signalling in Drosophila; dopamine does it all after all.Curr Opin Neurobiol. 2013 Jun;23(3):324-9. doi: 10.1016/j.conb.2013.01.005. Epub 2013 Feb 5. Curr Opin Neurobiol. 2013. PMID: 23391527 Free PMC article. Review.
-
Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior.Elife. 2018 Mar 16;7:e35264. doi: 10.7554/eLife.35264. Elife. 2018. PMID: 29547121 Free PMC article.
-
Representations of Novelty and Familiarity in a Mushroom Body Compartment.Cell. 2017 May 18;169(5):956-969.e17. doi: 10.1016/j.cell.2017.04.028. Epub 2017 May 11. Cell. 2017. PMID: 28502772 Free PMC article.
-
Changing memories on the fly: the neural circuits of memory re-evaluation in Drosophila melanogaster.Curr Opin Neurobiol. 2021 Apr;67:190-198. doi: 10.1016/j.conb.2020.12.003. Epub 2020 Dec 26. Curr Opin Neurobiol. 2021. PMID: 33373859 Review.
Cited by
-
Obesity- and diet-induced plasticity in systems that control eating and energy balance.Obesity (Silver Spring). 2024 Aug;32(8):1425-1440. doi: 10.1002/oby.24060. Epub 2024 Jul 15. Obesity (Silver Spring). 2024. PMID: 39010249 Free PMC article. Review.
-
Use-Dependent, Untapped Dual Kinase Signaling Localized in Brain Learning Circuitry.J Neurosci. 2024 Mar 20;44(12):e1126232024. doi: 10.1523/JNEUROSCI.1126-23.2024. J Neurosci. 2024. PMID: 38267256 Free PMC article.
-
The Neural Correlations of Olfactory Associative Reward Memories in Drosophila.Cells. 2024 Oct 17;13(20):1716. doi: 10.3390/cells13201716. Cells. 2024. PMID: 39451234 Free PMC article. Review.
-
The caloric value of food intake structurally adjusts a neuronal mushroom body circuit mediating olfactory learning in Drosophila.Learn Mem. 2024 Jun 11;31(5):a053997. doi: 10.1101/lm.053997.124. Print 2024 May. Learn Mem. 2024. PMID: 38862177 Free PMC article.
-
Skewing information flow through pre- and postsynaptic plasticity in the mushroom bodies of Drosophila.Learn Mem. 2024 Jun 14;31(5):a053919. doi: 10.1101/lm.053919.124. Print 2024 May. Learn Mem. 2024. PMID: 38876487 Free PMC article. Review.
References
-
- Benelam B (2009). Satiation, satiety and their effects on eating behaviour. Nutr. Bull 34, 126–173.
-
- Bellisle F, and Blundell JE (2013). Satiation, satiety: concepts and organisation of behaviour. Satiation, Satiety and the Control of Food Intake, 3–11. 10.1533/9780857098719.1.3. - DOI
-
- Davidson TL, and Martin AA (2014). Obesity: Cognitive impairment and the failure to “eat right.” Curr. Biol 24, R685–7. - PubMed
-
- Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V, et al. (2019). Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metabolism 30, 67–77.e3. 10.1016/j.cmet.2019.05.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

