The N-terminal domain of human mitochondrial helicase Twinkle has DNA-binding activity crucial for supporting processive DNA synthesis by polymerase γ
- PMID: 36528058
- PMCID: PMC9860392
- DOI: 10.1016/j.jbc.2022.102797
The N-terminal domain of human mitochondrial helicase Twinkle has DNA-binding activity crucial for supporting processive DNA synthesis by polymerase γ
Abstract
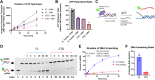
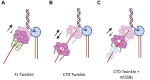
Twinkle is the ring-shaped replicative helicase within the human mitochondria with high homology to bacteriophage T7 gp4 helicase-primase. Unlike many orthologs of Twinkle, the N-terminal domain (NTD) of human Twinkle has lost its primase activity through evolutionarily acquired mutations. The NTD has no demonstrated activity thus far; its role has remained unclear. Here, we biochemically characterize the isolated NTD and C-terminal domain (CTD) with linker to decipher their contributions to full-length Twinkle activities. This novel CTD construct hydrolyzes ATP, has weak DNA unwinding activity, and assists DNA polymerase γ (Polγ)-catalyzed strand-displacement synthesis on short replication forks. However, CTD fails to promote multikilobase length product formation by Polγ in rolling-circle DNA synthesis. Thus, CTD retains all the motor functions but struggles to implement them for processive translocation. We show that NTD has DNA-binding activity, and its presence stabilizes Twinkle oligomerization. CTD oligomerizes on its own, but the loss of NTD results in heterogeneously sized oligomeric species. The CTD also exhibits weaker and salt-sensitive DNA binding compared with full-length Twinkle. Based on these results, we propose that NTD directly contributes to DNA binding and holds the DNA in place behind the central channel of the CTD like a "doorstop," preventing helicase slippages and sustaining processive unwinding. Consistent with this model, mitochondrial single-stranded DNA-binding protein (mtSSB) compensate for the NTD loss and partially restore kilobase length DNA synthesis by CTD and Polγ. The implications of our studies are foundational for understanding the mechanisms of disease-causing Twinkle mutants that lie in the NTD.
Keywords: DNA polymerase; Twinkle; helicase; mitochondria; mitochondrial diseases; replication; replisome.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







Similar articles
-
The Dictyostelium discoideum homologue of Twinkle, Twm1, is a mitochondrial DNA helicase, an active primase and promotes mitochondrial DNA replication.BMC Mol Biol. 2018 Dec 19;19(1):12. doi: 10.1186/s12867-018-0114-7. BMC Mol Biol. 2018. PMID: 30563453 Free PMC article.
-
The N-terminal domain of TWINKLE contributes to single-stranded DNA binding and DNA helicase activities.Nucleic Acids Res. 2008 Feb;36(2):393-403. doi: 10.1093/nar/gkm1025. Epub 2007 Nov 26. Nucleic Acids Res. 2008. PMID: 18039713 Free PMC article.
-
The plant organellar primase-helicase directs template recognition and primosome assembly via its zinc finger domain.BMC Plant Biol. 2023 Oct 6;23(1):467. doi: 10.1186/s12870-023-04477-4. BMC Plant Biol. 2023. PMID: 37803262 Free PMC article.
-
Mechanisms of a ring shaped helicase.Nucleic Acids Res. 2006;34(15):4216-24. doi: 10.1093/nar/gkl508. Epub 2006 Aug 25. Nucleic Acids Res. 2006. PMID: 16935879 Free PMC article. Review.
-
TWINKLE and Other Human Mitochondrial DNA Helicases: Structure, Function and Disease.Genes (Basel). 2020 Apr 9;11(4):408. doi: 10.3390/genes11040408. Genes (Basel). 2020. PMID: 32283748 Free PMC article. Review.
Cited by
-
Autoregulation of the real-time kinetics of the human mitochondrial replicative helicase.Nat Commun. 2025 Jul 1;16(1):5460. doi: 10.1038/s41467-025-60289-0. Nat Commun. 2025. PMID: 40592829 Free PMC article.
-
Twinkle-Catalyzed Toehold-Mediated DNA Strand Displacement Reaction.J Am Chem Soc. 2023 Nov 2:10.1021/jacs.3c04970. doi: 10.1021/jacs.3c04970. Online ahead of print. J Am Chem Soc. 2023. PMID: 37917930 Free PMC article.
-
Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions.Front Endocrinol (Lausanne). 2024 Apr 17;15:1361289. doi: 10.3389/fendo.2024.1361289. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38694941 Free PMC article. Review.
References
-
- Spelbrink J.N., Li F.Y., Tiranti V., Nikali K., Yuan Q.P., Tariq M., et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 2001;28:223–231. - PubMed
-
- Remtulla S., Emilie Nguyen C.T., Prasad C., Campbell C. Twinkle-associated mitochondrial DNA depletion. Pediatr. Neurol. 2019;90:61–65. - PubMed
-
- Pierce S.B., Gulsuner S., Stapleton G.A., Walsh T., Lee M.K., Mandell J.B., et al. Infantile onset spinocerebellar ataxia caused by compound heterozygosity for Twinkle mutations and modeling of Twinkle mutations causing recessive disease. Cold Spring Harb Mol. Case Stud. 2016;2:a001107. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

