Cholinergic control of Th17 cell pathogenicity in experimental autoimmune encephalomyelitis
- PMID: 36528755
- PMCID: PMC9950465
- DOI: 10.1038/s41418-022-01092-y
Cholinergic control of Th17 cell pathogenicity in experimental autoimmune encephalomyelitis
Abstract
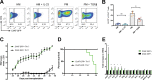
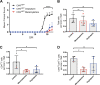
Experimental autoimmune encephalomyelitis (EAE) is a mouse model of multiple sclerosis (MS) in which Th17 cells have a crucial but unclear function. Here we show that choline acetyltransferase (ChAT), which synthesizes acetylcholine (ACh), is a critical driver of pathogenicity in EAE. Mice with ChAT-deficient Th17 cells resist disease progression and show reduced brain-infiltrating immune cells. ChAT expression in Th17 cells is linked to strong TCR signaling, expression of the transcription factor Bhlhe40, and increased Il2, Il17, Il22, and Il23r mRNA levels. ChAT expression in Th17 cells is independent of IL21r signaling but dampened by TGFβ, implicating ChAT in controlling the dichotomous nature of Th17 cells. Our study establishes a cholinergic program in which ACh signaling primes chronic activation of Th17 cells, and thereby constitutes a pathogenic determinant of EAE. Our work may point to novel targets for therapeutic immunomodulation in MS.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors do not have competing financial interests concerning the work described. T. Mak owns equity in Treadwell Therapeutics Inc. and Agios Pharmaceuticals and is a consultant for AstraZeneca and Tessa Therapeutics.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

