Blood-stage antiplasmodial activity and oocyst formation-blockage of metallo copper-cinchonine complex
- PMID: 36530433
- PMCID: PMC9751060
- DOI: 10.3389/fcimb.2022.1047269
Blood-stage antiplasmodial activity and oocyst formation-blockage of metallo copper-cinchonine complex
Abstract
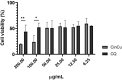
In the fight against malaria, the key is early treatment with antimalarial chemotherapy, such as artemisinin-based combination treatments (ACTs). However, Plasmodium has acquired multidrug resistance, including the emergence of P. falciparum strains with resistance to ACT. The development of novel antimalarial molecules, that are capable of interfering in the asexual and sexual blood stages, is important to slow down the transmission in endemic areas. In this work, we studied the ability of the mettalo copper-cinchonine complex to interfere in the sexual and asexual stages of Plasmodium. The tested compound in the in vitro assay was a cinchonine derivative, named CinCu (Bis[Cinchoninium Tetrachlorocuprate(II)]trihydrate). Its biological functions were assessed by antiplasmodial activity in vitro against chloroquine-resistant P. falciparum W2 strain. The mice model of P. berghei ANKA infection was used to analyze the antimalarial activity of CinCu and chloroquine and their acute toxicity. The oocyst formation-blocking assay was performed by experimental infection of Anopheles aquasalis with P. vivax infected blood, which was treated with different concentrations of CinCu, cinchonine, and primaquine. We found that CinCu was able to suppress as high as 81.58% of parasitemia in vitro, being considered a molecule with high antiplasmodial activity and low toxicity. The in vivo analysis showed that CinCu suppressed parasitemia at 34% up to 87.19%, being a partially active molecule against the blood-stage forms of P. berghei ANKA, without inducing severe clinical signs in the treated groups. The transmission-blocking assay revealed that both cinchonine and primaquine were able to reduce the infection intensity of P. vivax in A. aquasalis, leading to a decrease in the number of oocysts recovered from the mosquitoes' midgut. Regarding the effect of CinCu, the copper-complex was not able to induce inhibition of P. vivax infection; however, it was able to induce an important reduction in the intensity of oocyst formation by about 2.4 times. It is plausible that the metallo-compound also be able to interfere with the differentiation of parasite stages and/or ookinete-secreted chitinase into the peritrophic matrix of mosquitoes, promoting a reduction in the number of oocysts formed. Taken together, the results suggest that this compound is promising as a prototype for the development of new antimalarial drugs. Furthermore, our study can draw a new pathway for repositioning already-known antimalarial drugs by editing their chemical structure to improve the antimalarial activity against the asexual and sexual stages of the parasite.
Keywords: Plasmodium; antimalarial drugs; cinchonine; malaria treatment; mettalo copper complexes.
Copyright © 2022 Morais, Brito, Weselucha-Birczyńska, Pereira, Pereira-Silva, Menezes, Pessoa, Kucharska, Birczyńska-Zych, Ríos-Velásquez and Andrade-Neto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Andrade-Neto V. F. (2000). Atividade antimalárica de extratos brutos, frações semi-purificadas e de compostos quimicamente definidos isolados de plantas utilizadas Na medicina popular (Belo Horizonte (MG: Federal University of Minas Gerais; ).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

