Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom
- PMID: 36530491
- PMCID: PMC9742675
- DOI: 10.1016/j.lanepe.2022.100556
Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom
Abstract
Background: There is an urgent public health need to evaluate disease severity in adults hospitalised with Delta and Omicron SARS-CoV-2 variant infections. However, limited data exist assessing severity of disease in adults hospitalised with Omicron SARS-CoV-2 infections, and to what extent patient-factors, including vaccination, age, frailty and pre-existing disease, affect variant-dependent disease severity.
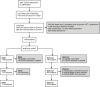
Methods: A prospective cohort study of adults (≥18 years of age) hospitalised with acute lower respiratory tract disease at acute care hospitals in Bristol, UK conducted over 10-months. Delta or Omicron SARS-CoV-2 infection was defined by positive SARS-CoV-2 PCR and variant identification or inferred by dominant circulating variant. We constructed adjusted regression analyses to assess disease severity using three different measures: FiO2 >28% (fraction inspired oxygen), World Health Organization (WHO) outcome score >5 (assessing need for ventilatory support), and hospital length of stay (LOS) >3 days following admission for Omicron or Delta infection.
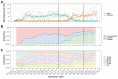
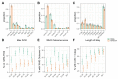
Findings: Independent of other variables, including vaccination, Omicron variant infection in hospitalised adults was associated with lower severity than Delta. Risk reductions were 58%, 67%, and 16% for supplementary oxygen with >28% FiO2 [Relative Risk (RR) = 0.42 (95%CI: 0.34-0.52), P < 0.001], WHO outcome score >5 [RR = 0.33 (95%CI: 0.21-0.50), P < 0.001], and to have had a LOS > 3 days [RR = 0.84 (95%CI: 0.76-0.92), P < 0.001]. Younger age and vaccination with two or three doses were also independently associated with lower COVID-19 severity.
Interpretation: We provide reassuring evidence that Omicron infection results in less serious adverse outcomes than Delta in hospitalised patients. Despite lower severity relative to Delta, Omicron infection still resulted in substantial patient and public health burden and an increased admission rate of older patients with Omicron which counteracts some of the benefit arising from less severe disease.
Funding: AvonCAP is an investigator-led project funded under a collaborative agreement by Pfizer.
Keywords: COVID-19; Respiratory infection; SARS-CoV-2; Vaccination.
© 2022 The Author(s).
Conflict of interest statement
CH is Principal Investigator of the AvonCAP study which is an investigator-led University of Bristol study funded by Pfizer and has previously received support from the NIHR in an Academic Clinical Fellowship. JO is a Co-Investigator on the AvonCAP Study. AF is a member of the Joint Committee on Vaccination and Immunization (JCVI) and chair of the World Health Organization European Technical Advisory Group of Experts on Immunization (ETAGE) committee. In addition to receiving funding from Pfizer as Chief Investigator of this study, he leads another project investigating transmission of respiratory bacteria in families jointly funded by Pfizer and the Gates Foundation. LD, RC are members of SPI-M-O subgroups of SAGE and are also partly funded through AvonCAP. LD is a Co-Investigator of the AvonCAP study and has also received funding from Pfizer, UKRI and UKHSA for unrelated projects. EB, JS, JN, SG, GE, LJ, BG, and JMM are employees of Pfizer, Inc and may hold stock or stock options. The other authors have no relevant conflicts of interest to declare.
Figures

References
-
- Challen R., Dyson L., Overton C.E., et al. Early epidemiological signatures of novel SARS-CoV-2 variants: establishment of B.1.617.2 in England. medRxiv. 2021 doi: 10.1101/2021.06.05.21258365. - DOI
-
- Government U Coronavirus (COVID-19) in the UK. 2022. https://coronavirus.data.gov.uk/details/vaccinations?areaType=overview&a...
-
- UKHSA. SARS-CoV-2 variants of concern and variants under investigation in England, technical briefing 36. 2022.
-
- Wang P., Nair M.S., Liu L., et al. Antibody resistance of SARS-CoV-2 variants B. 1.351 and B. 1.1. 7. Nature. 2021;593(7857):130–135. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

