Early intervention in psoriasis: Where do we go from here?
- PMID: 36530901
- PMCID: PMC9751903
- DOI: 10.3389/fmed.2022.1027347
Early intervention in psoriasis: Where do we go from here?
Abstract
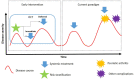
Patients with psoriasis often have comorbidities and are at increased risk of developing several complications compared with the general population. Knowledge on the role of immune mediators and systemic inflammation in psoriasis has led to the hypothesis that early intervention with systemic therapy has the potential to modify the course of the disease and reduce the risk of long-term adverse outcomes. In this article, we address some potential issues that need to be considered before early intervention can be implemented routinely. The first is determining what constitutes "early" intervention for psoriasis. A second point is whether the intervention should be considered for patients with early disease or for selected subsets based on risk stratification. A third important consideration is defining success for early intervention. Finally, adoption of early and effective intervention should be based on high-level evidence. Ideally, randomized trials would be the best strategy to compare early vs. late systemic treatment in patients with psoriasis, probably using the frequency of long-term outcomes as primary endpoint, with cutaneous and pharmacoeconomic outcomes assessed secondarily.
Keywords: early intervention; methotrexate; psoriasis; risk stratification; systemic treatment; therapeutic success.
Copyright © 2022 Felix, Sampaio, Silva and Viana.
Conflict of interest statement
PF has served as investigator and/or consultant to advisory boards and as paid speaker for AbbVie, Amgen, Boehring Ingelheim, Ely Lilly, Janssen, Leopharma, Novartis, Pfizer, Sandoz, Sanofi, and UCB. AS has served as consultant to advisory boards for Novartis and Janssen, and paid speaker for Novartis, Janssen, and Leopharma. AV is a former employee of AbbVie and may own AbbVie stock or stock options. BS is an employee of AbbVie and may own AbbVie stock or stock options. The authors declare that this study received funding from AbbVie. The funder participated in the interpretation of data and the review and approval of the content.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources