Can urine studies be replaced by serum free light chains measurements to assign responses in multiple myeloma patients?
- PMID: 36531081
- PMCID: PMC9748693
- DOI: 10.3389/fonc.2022.1056293
Can urine studies be replaced by serum free light chains measurements to assign responses in multiple myeloma patients?
Abstract
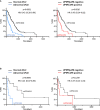
Serum and urine protein electrophoresis and immunofixation are the preferred techniques for monitoring monoclonal proteins and evaluating treatment response in multiple myeloma (MM) patients with measurable disease. However, urine studies are subjected to limitations that may lead to inaccuracies or prevent guidelines compliance. We retrospectively studied if the substitution of urine studies by measuring serum free light chains (sFLCs) results in a comparable disease monitoring, both in intact immunoglobulin (II) and light chain (LC) MM patients. In our cohort, equal or higher percentages of disease were identified by sFLCs at baseline and maximum response as compared to urine studies. Achieving very good partial response or better (≥VGPR) according to the response criteria proposed by the French group (evaluating sFLCs instead of urine) and the IMWG response criteria were associated to a 62% and 63% reduced risk of progression, respectively. A similar prognostic value for reaching ≥VGPR was also observed among LCMM patients when the French group and the IMWG response criteria were applied. Overall, these results support the replacement of urine studies by the sFLCs assay in IIMM. In LCMM, sFLCs could be used for monitoring and urine studies could be performed only to confirm complete remissions and progressions.
Keywords: multiple myeloma; response criteria; serum free light chains; urine protein electrophoresis; urine protein immunofixation.
Copyright © 2022 Cárdenas, Iñigo, Ortega, Palomar, Menéndez, Plaza, Martínez-Novillo and Benavente.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

