The impact of multi-target stool DNA testing in clinical practice in the United States: A real-world evidence retrospective study
- PMID: 36531100
- PMCID: PMC9747652
- DOI: 10.1016/j.pmedr.2022.102045
The impact of multi-target stool DNA testing in clinical practice in the United States: A real-world evidence retrospective study
Abstract
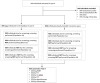
Widely endorsed screening modalities for colorectal cancer (CRC) include structural visualization (e.g. colonoscopy) and stool-based tests including multitarget stool DNA (mt-sDNA), fecal immunochemical tests (FIT), or high-sensitivity guaiac-based fecal occult blood tests (gFOBT). However, CRC screenings are underutilized, hence understanding the screening utilization trends is important, particularly with respect to the newest guideline-endorsed option (mt-sDNA). The objective of this study was to assess patterns in overall CRC screenings following clinical availability of the mt-sDNA test among average-risk individuals in the Ascension Wisconsin healthcare system focusing primarily on individuals aged 50-75 years old. We also reported CRC screening behaviors among individuals < 50 and > 75 years old. Electronic medical records of individuals aged ≥ 40 years from 2015 to 2018 were reviewed to identify average-risk and screen-eligible members. For those with screening data available, we determined the proportion who were up-to-date with any United States Preventive Services Task Force (USPSTF) recommended screening strategy; the number of screening tests performed in the measurement year; and the distribution of screening modalities. Temporal trends were assessed using regression analysis, including subgroup analyses across age groups and screening modalities. A total of 172,045 unique patients aged ≥ 40 years were included, of which 115,708 individuals aged 50-75 years. When considering all individuals up-to-date and screened in the measurement year, overall adherence increased significantly over the 4-year study period, from 39,105 to 49,698 patients or 47 % to 59 % (p < 0.0001). The screening incidence between 2015 and 2018 increased from 19.44 to 23.66 tests per 1,000 persons for gFOBT and FIT, a 1.2-fold increase, and from 6.54 to 29.78 tests per 1,000 persons for mt-sDNA (p < 0.05), a 4.6-fold increase. During the same time period, the screening incidence of colonoscopy decreased from 119.99 to 110.58 tests per 1,000 persons, corresponding to a decrease of 8 %. Similar patterns in screening incidence rates were observed among those aged < 50 and > 75 years old. Growing adoption, higher preference, and the broad availability of mt-sDNA testing may be associated with an increase in overall CRC screening rates in the average-risk population, in parallel with a slight increase in the use of other non-invasive CRC screening tests.
Keywords: Colonoscopy Stool-based tests; Colorectal Cancer; Real-World; Screening.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Healthcare costs, resource utilization, and productivity loss associated with colorectal cancer screening.Expert Rev Pharmacoecon Outcomes Res. 2023 Jul-Dec;23(7):843-852. doi: 10.1080/14737167.2023.2220965. Epub 2023 Jul 18. Expert Rev Pharmacoecon Outcomes Res. 2023. PMID: 37462667
-
The cost-effectiveness of non-invasive stool-based colorectal cancer screening offerings from age 45 for a commercial and medicare population.J Med Econ. 2023 Jan-Dec;26(1):1219-1226. doi: 10.1080/13696998.2023.2260681. Epub 2023 Oct 6. J Med Econ. 2023. PMID: 37752872
-
Barriers to utilization of three colorectal cancer screening options - Data from a national survey.Prev Med Rep. 2021 Jul 29;24:101508. doi: 10.1016/j.pmedr.2021.101508. eCollection 2021 Dec. Prev Med Rep. 2021. PMID: 34401220 Free PMC article.
-
Multitarget stool DNA for colorectal cancer screening: A review and commentary on the United States Preventive Services Draft Guidelines.World J Gastrointest Oncol. 2016 May 15;8(5):450-8. doi: 10.4251/wjgo.v8.i5.450. World J Gastrointest Oncol. 2016. PMID: 27190584 Free PMC article. Review.
-
Noninvasive fecal testing for colorectal cancer.Clin Chim Acta. 2022 Jan 1;524:123-131. doi: 10.1016/j.cca.2021.10.030. Epub 2021 Oct 28. Clin Chim Acta. 2022. PMID: 34756863 Review.
Cited by
-
Attitudes and Experiential Factors Associated with Completion of mt-sDNA Test Kit for Colorectal Cancer Screening.J Patient Exp. 2023 Nov 22;10:23743735231213765. doi: 10.1177/23743735231213765. eCollection 2023. J Patient Exp. 2023. PMID: 38026067 Free PMC article.
-
Colonoscopy Remains an Important Option for Primary Screening for Colorectal Cancer.Dig Dis Sci. 2025 May;70(5):1595-1605. doi: 10.1007/s10620-024-08760-8. Epub 2024 Dec 12. Dig Dis Sci. 2025. PMID: 39666212 Review.
-
Opportunities and Challenges in Screening for Colorectal Cancer.Popul Health Manag. 2023 Aug;26(4):246-253. doi: 10.1089/pop.2023.0013. Epub 2023 Jul 26. Popul Health Manag. 2023. PMID: 37498933 Free PMC article.
-
Adherence to Follow-Up Colonoscopy After a Positive Stool-Based Test in Patients Aged 45-49 Years: Real-World Differences Between Multitarget Stool DNA Testing and Fecal Immunochemical or Fecal Occult Blood Testing.Gastro Hep Adv. 2025 May 16;4(9):100706. doi: 10.1016/j.gastha.2025.100706. eCollection 2025. Gastro Hep Adv. 2025. PMID: 40688388 Free PMC article.
-
Cost-Effectiveness of Liquid Biopsy for Colorectal Cancer Screening in Patients Who Are Unscreened.JAMA Netw Open. 2023 Nov 1;6(11):e2343392. doi: 10.1001/jamanetworkopen.2023.43392. JAMA Netw Open. 2023. PMID: 37971743 Free PMC article.
References
-
- American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Atlanta: American Cancer Society; 2020.
-
- American Cancer Society, 2021. Key Statistics for Colorectal Cancer: How common is colorectal cancer? Available at https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics. Accessed March 16, 2022.
-
- BRFSS Survey Data and Documentation, 2020. Behavioral Risk Factor Surveillance System. Atlanta : Centers for Disease Control and Prevention, 2020.
-
- Corley D.A., Jensen C.D., Quinn V.P., Doubeni C.A., Zauber A.G., Lee J.K., Schottinger J.E., Marks A.R., Zhao W.K., Ghai N.R., Lee A.T., Contreras R., Quesenberry C.P., Fireman B.H., Levin T.R. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA. 2017;317(16):1631–1641. doi: 10.1001/jama.2017.3634. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources