Cerebral multimodality monitoring in adult neurocritical care patients with acute brain injury: A narrative review
- PMID: 36531179
- PMCID: PMC9751622
- DOI: 10.3389/fphys.2022.1071161
Cerebral multimodality monitoring in adult neurocritical care patients with acute brain injury: A narrative review
Abstract
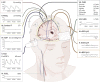
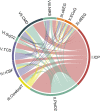
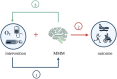
Cerebral multimodality monitoring (MMM) is, even with a general lack of Class I evidence, increasingly recognized as a tool to support clinical decision-making in the neuroscience intensive care unit (NICU). However, literature and guidelines have focused on unimodal signals in a specific form of acute brain injury. Integrating unimodal signals in multiple signal monitoring is the next step for clinical studies and patient care. As such, we aimed to investigate the recent application of MMM in studies of adult patients with traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), acute ischemic stroke (AIS), and hypoxic ischemic brain injury following cardiac arrest (HIBI). We identified continuous or daily updated monitoring modalities and summarized the monitoring setting, study setting, and clinical characteristics. In addition, we discussed clinical outcome in intervention studies. We identified 112 MMM studies, including 11 modalities, over the last 7 years (2015-2022). Fifty-eight studies (52%) applied only two modalities. Most frequently combined were ICP monitoring (92 studies (82%)) together with PbtO2 (63 studies (56%). Most studies included patients with TBI (59 studies) or SAH (53 studies). The enrollment period of 34 studies (30%) took more than 5 years, whereas the median sample size was only 36 patients (q1- q3, 20-74). We classified studies as either observational (68 studies) or interventional (44 studies). The interventions were subclassified as systemic (24 studies), cerebral (10 studies), and interventions guided by MMM (11 studies). We identified 20 different systemic or cerebral interventions. Nine (9/11, 82%) of the MMM-guided studies included clinical outcome as an endpoint. In 78% (7/9) of these MMM-guided intervention studies, a significant improvement in outcome was demonstrated in favor of interventions guided by MMM. Clinical outcome may be improved with interventions guided by MMM. This strengthens the belief in this application, but further interdisciplinary collaborations are needed to overcome the heterogeneity, as illustrated in the present review. Future research should focus on increasing sample sizes, improved data collection, refining definitions of secondary injuries, and standardized interventions. Only then can we proceed with complex outcome studies with MMM-guided treatment.
Keywords: AIS; HIBI; ICH; SAH; TBI; cerebral multimodality monitoring; intensive care; outcome.
Copyright © 2022 Tas, Czosnyka, van der Horst, Park, van Heugten, Sekhon, Robba, Menon, Zeiler and Aries.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Albanna W., Weiss M., Müller M., Brockmann M. A., Rieg A., Conzen C., et al. (2017). Endovascular rescue therapies for refractory vasospasm after subarachnoid hemorrhage: A prospective evaluation study using multimodal, continuous event neuromonitoring. Neurosurgery 80 (6), 942–949. 10.1093/neuros/nyw132 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

