Anticancer effect of zinc oxide nanoparticles prepared by varying entry time of ion carriers against A431 skin cancer cells in vitro
- PMID: 36531331
- PMCID: PMC9751667
- DOI: 10.3389/fchem.2022.1069450
Anticancer effect of zinc oxide nanoparticles prepared by varying entry time of ion carriers against A431 skin cancer cells in vitro
Abstract
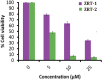
Although, zinc oxide nanoparticles (ZRTs) as an anti-cancer agent have been the subject of numerous studies, none of the reports has investigated the impact of the reaction entry time of ion-carriers on the preparation of ZRTs. Therefore, we synthesized variants of ZRTs by extending the entry time of NaOH (that acts as a carrier of hydroxyl ions) in the reaction mixture. The anti-proliferative action, morphological changes, reactive oxygen species (ROS) production, and nuclear apoptosis of ZRTs on human A431 skin carcinoma cells were observed. The samples revealed crystallinity and purity by X-ray diffraction (XRD). Scanning electron microscopy (SEM) images of ZRT-1 (5 min ion carrier entry) and ZRT-2 (10 min ion carrier entry) revealed microtubule like morphology. On prolonging the entry time for ion carrier (NaOH) introduction in the reaction mixture, a relative ascent in the aspect ratio was seen. The typical ZnO band with a slight shift in the absorption maxima was evident with UV-visible spectroscopy. Both ZRT-1 and ZRT-2 exhibited non-toxic behavior as evident by RBC lysis assay. Additionally, ZRT-2 showed better anti-cancer potential against A431 cells as seen by MTT assay, ROS generation and chromatin condensation analyses. At 25 μM of ZRT-2, 5.56% cells were viable in MTT test, ROS production was enhanced to 166.71%, while 33.0% of apoptotic cells were observed. The IC50 for ZRT-2 was slightly lower (6 μM) than that for ZRT-1 (8 μM) against A431 cells. In conclusion, this paper presents a modest, economical procedure to generate ZRT nano-structures exhibiting strong cytotoxicity against the A431 cell line, indicating that ZRTs may have application in combating cancer.
Keywords: MTT assay; malignant cell lines; reactive oxygen species; sol-gel synthesis; zinc oxide nanoparticles.
Copyright © 2022 Aljohar, Muteeb, Zia, Siddiqui, Aatif, Farhan, Khan, Alsultan, Jamal, Alshoaibi, Ahmad, Alam, Arshad and Ahamed.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Husk-like Zinc Oxide Nanoparticles Induce Apoptosis through ROS Generation in Epidermoid Carcinoma Cells: Effect of Incubation Period on Sol-Gel Synthesis and Anti-Cancerous Properties.Biomedicines. 2023 Jan 23;11(2):320. doi: 10.3390/biomedicines11020320. Biomedicines. 2023. PMID: 36830857 Free PMC article.
-
Zinc Oxide Nanoparticle Induces Apoptosis in Human Epidermoid Carcinoma Cells Through Reactive Oxygen Species and DNA Degradation.Biol Trace Elem Res. 2021 Jun;199(6):2172-2181. doi: 10.1007/s12011-020-02323-4. Epub 2020 Aug 25. Biol Trace Elem Res. 2021. PMID: 32840725
-
Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential.Int J Nanomedicine. 2020 Nov 2;15:8519-8536. doi: 10.2147/IJN.S271743. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33173290 Free PMC article.
-
Ultrastructural analysis of zinc oxide nanospheres enhances anti-tumor efficacy against Hepatoma.Front Oncol. 2022 Oct 27;12:933750. doi: 10.3389/fonc.2022.933750. eCollection 2022. Front Oncol. 2022. PMID: 36457501 Free PMC article.
-
Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism.Adv Colloid Interface Sci. 2017 Nov;249:37-52. doi: 10.1016/j.cis.2017.07.033. Epub 2017 Aug 26. Adv Colloid Interface Sci. 2017. PMID: 28923702 Review.
Cited by
-
Sustainable fabrication of dimorphic plant derived ZnO nanoparticles and exploration of their biomedical and environmental potentialities.Sci Rep. 2024 Jun 12;14(1):13459. doi: 10.1038/s41598-024-63459-0. Sci Rep. 2024. PMID: 38862646 Free PMC article.
-
Exploring the multi-faceted potential: Synthesized ZnO nanostructure - Characterization, photocatalysis, and crucial biomedical applications.Heliyon. 2024 Jun 13;10(12):e32714. doi: 10.1016/j.heliyon.2024.e32714. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39022102 Free PMC article.
-
Zinc oxide nanoparticles derived from Penicillium griseofulvum mitigate DMBA/TPA-promoted mice skin carcinogenesis by modulating NF-ĸB associated signalling.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):891-902. doi: 10.1007/s00210-024-03311-4. Epub 2024 Jul 30. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39080011
-
Design, Synthesis, and Characterization of Novel Pyrazole Cross-Linked Chitosan Derivatives Modified with Zinc Oxide Nanoparticles for Boosting Their Anticancer Activity.Polymers (Basel). 2025 Apr 15;17(8):1061. doi: 10.3390/polym17081061. Polymers (Basel). 2025. PMID: 40284326 Free PMC article.
-
Husk-like Zinc Oxide Nanoparticles Induce Apoptosis through ROS Generation in Epidermoid Carcinoma Cells: Effect of Incubation Period on Sol-Gel Synthesis and Anti-Cancerous Properties.Biomedicines. 2023 Jan 23;11(2):320. doi: 10.3390/biomedicines11020320. Biomedicines. 2023. PMID: 36830857 Free PMC article.
References
-
- Abbasi B. H., Shah M., Hashmi S. S., Nazir M., Naz S., Ahmad W., et al. (2019). Green bio-assisted synthesis, characterization and biological evaluation of biocompatible ZnO NPs synthesized from different tissues of milk thistle (Silybum marianum). Nanomater. (Basel). 9, 1171. 10.3390/nano9081171 - DOI - PMC - PubMed
-
- Adam R. E., ’ Pozina G., Willander M., Nur O. (2018). Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostructures - Fundam. Appl. 32, 11–18. 10.1016/j.photonics.2018.08.005 - DOI
-
- Akintelu S. A., Folorunso A. S. (2020). A review on green synthesis of zinc oxide nanoparticles using plant extracts and its biomedical applications. BioNanoScience 10, 848–863. 10.1007/s12668-020-00774-6 - DOI
LinkOut - more resources
Full Text Sources

