Natural Course of Pachychoroid Pigment Epitheliopathy
- PMID: 36531587
- PMCID: PMC9754975
- DOI: 10.1016/j.xops.2022.100201
Natural Course of Pachychoroid Pigment Epitheliopathy
Abstract
Purpose: To investigate the natural course of pachychoroid pigment epitheliopathy (PPE).
Design: A retrospective cohort study.
Subjects: From the Kyoto central serous chorioretinopathy (CSC) cohort consisting of 548 patients with CSC as of September 2020, we included consecutive unilateral patients with acute or chronic CSC between January 2013 and December 2016.
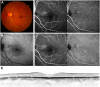
Methods: All patients underwent complete ophthalmic examination, including multimodal imaging such as fundus autofluorescence, spectral-domain optical coherence tomography, and fluorescein angiography/indocyanine green angiography and/or optimal coherence tomography angiography. The fellow eyes of eyes diagnosed with CSC were screened for PPE, and their natural course was evaluated. We also evaluated the association of ARMS2 rs10490924, CFH rs800292, TNFRSF10A rs13278062, and GATA5 rs6061548 genotypes with the natural course.
Main outcome measures: Incidence of CSC, pachychoroid neovasculopathy, and pachychoroid geographic atrophy (GA).
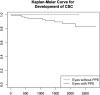
Results: In total, 165 patients with unilateral CSC (mean age, 55.7 ± 12.6 years; female, 22.4%) were included from the Kyoto CSC cohort. Among them, 148 (89.7%) were diagnosed as having PPE in their non-CSC eye. Survival analysis revealed that 16.8% of PPE eyes developed CSC during the 6-year follow up, whereas non-PPE eyes did not. Although genetic factors did not have significant association with CSC development (P > 0.05, log-rank test), choroidal vascular hyperpermeability (CVH) and subfoveal choroidal thickness (SFCT) were significantly associated with CSC incidence (P = 0.001, log-rank test). Survival analysis showed that eyes without CVH and eyes with SFCT < 300 μm did not develop CSC during the 6-year follow-up. Pachychoroid neovasculopathy developed in only 1 eye with PPE during a follow-up of 46.4 months. Pachychoroid GA did not develop in any of the studied eyes.
Conclusions: This study revealed a natural history of PPE in a relatively large Japanese cohort. Choroidal vascular hyperpermeability and SFCT were significant risk factors for the development of CSC in PPE eyes. Although the current results cannot be generalized for all eyes with PPE, these findings present an important clinical implication.
Keywords: BCVA, best-corrected visual acuity; CI, confidence interval; CSC, central serous chorioretinopathy; CVH, choroidal vascular hyperpermeability; FA, fluorescein angiography; GA, geographic atrophy; ICGA, indocyanine green angiography; MNV, macular neovascularization; PNV, pachychoroid neovasculopathy; PPE, pachychoroid pigment epitheliopathy; SD, standard deviation; SFCT, Subfoveal choroidal thickness; SNPs, single nucleotide polymorphisms; central serous chorioretinopathy; pachychoroid; pachychoroid pigment epitheliopathy.
© 2022 by the American Academy of Ophthalmology.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

