Von Willebrand factor in diagnostics and treatment of cardiovascular disease: Recent advances and prospects
- PMID: 36531725
- PMCID: PMC9755348
- DOI: 10.3389/fcvm.2022.1038030
Von Willebrand factor in diagnostics and treatment of cardiovascular disease: Recent advances and prospects
Abstract
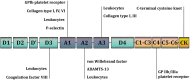
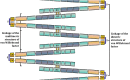
Von Willebrand factor (VWF) is a large multimeric glycoprotein involved in hemostasis. It is essential for platelet adhesion to the subendothelium of the damaged endothelial layer at high shear rates. Such shear rates occur in small-diameter arteries, especially at stenotic sites. Moreover, VWF carries coagulation factor VIII and protects it from proteolysis in the bloodstream. Deficiency or dysfunction of VWF predisposes to bleeding. In contrast, an increase in the concentration of high molecular weight multimers (HMWM) of VWF is closely associated with arterial thrombotic events. Severe aortic stenosis (AS) or hypertrophic obstructive cardiomyopathy (HOCM) can deplete HMWM of VWF and lead to cryptogenic, gastrointestinal, subcutaneous, and mucosal bleeding. Considering that VWF facilitates primary hemostasis and a local inflammatory response at high shear rates, its dysfunction may contribute to the development of coronary artery disease (CAD) and its complications. However, current diagnostic methods do not allow for an in-depth analysis of this contribution. The development of novel diagnostic techniques, primarily microfluidic, is underway. Such methods can provide physiologically relevant assessments of VWF function at high shear rates; however, they have not been introduced into clinical practice. The development and use of agents targeting VWF interaction with the vessel wall and/or platelets may be reasonable in prevention of CAD and its complications, given the prominent role of VWF in arterial thrombosis.
Keywords: ADAMTS-13; Heyde syndrome; cardiovascular disease; coronary artery disease; von Willebrand factor.
Copyright © 2022 Kozlov, Okhota, Avtaeva, Melnikov, Matroze and Gabbasov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

