The serotonin receptor 5-HT2A modulates lifespan and protein feeding in Drosophila melanogaster
- PMID: 36531741
- PMCID: PMC9751412
- DOI: 10.3389/fragi.2022.1068455
The serotonin receptor 5-HT2A modulates lifespan and protein feeding in Drosophila melanogaster
Abstract
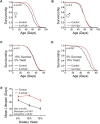
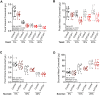
The conserved neurotransmitter serotonin has been shown to be an important modulator of lifespan in specific nutritional contexts; however, it remained unclear how serotonin signaling influences lifespan under normal conditions. Here, we show that serotonin signaling through the 5-HT2A receptor influences lifespan, behavior, and physiology in Drosophila. Loss of the 5-HT2A receptor extends lifespan and induces a resistance to changes in dietary protein that are normally detrimental to lifespan. 5-HT2A -/- null mutant flies also display decreased protein feeding and protein content in the body. Therefore, serotonin signaling through receptor 5-HT2A is likely recruited to promote motivation for protein intake, and chronic reduction of protein-drive through loss of 5-HT2A signaling leads to a lower protein set-point adaptation, which influences physiology, decreases feeding, and increases lifespan. Our findings reveal insights into the mechanisms by which organisms physiologically adapt in response to perceived inability to satisfy demand.
Keywords: Drosophila melanogaster; genetics; lifespan; nutrients; protein; serotonin.
Copyright © 2022 Munneke, Chakraborty, Porter, Gendron and Pletcher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Anisimov V. N., Berstein L. M., Egormin P. A., Piskunova T. S., Popovich I. G., Zabezhinski M. A., et al. (2005). Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp. Gerontol. 40 (8-9), 685–693. 10.1016/j.exger.2005.07.007 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

