End-Of-Life Care in the Potential Donor after Circulatory Death: A Systematic Review
- PMID: 36531837
- PMCID: PMC9755608
- DOI: 10.1177/19418744221123194
End-Of-Life Care in the Potential Donor after Circulatory Death: A Systematic Review
Abstract
Background: Donation after circulatory death (DCD) is becoming increasingly common, yet little is known about the way potential donors receive end-of-life care.
Purpose: The aims of this systematic review are to describe the current practice in end-of-life care for potential donors and identify metrics that are being used to assess discomfort among these patients.
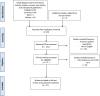
Research design and study sample: This review encompasses published literature between June 1, 2000 and June 31, 2020 of end-of-life care received by potential DCD patients. The population of interest was defined as patients eligible for Maastracht classification III donation after circulatory death for a solid organ transplantation. Outcomes examined included: analgesic or palliative protocols, and surrogates of discomfort (eg dyspnea, agitation).
Results: Among 141 unique articles, 27 studies were included for full review. The primary reason for exclusion was lack of protocol description, or lack of reporting on analgesic medications. No primary research studies specifically examined distress in the DCD eligible population. Numerous professional guidelines were identified. Surveys of critical care practitioners identified concerns regarding the impact of symptom management on hastening the dying process in the DCD population as a potential barrier to end-of-life palliative treatment.
Conclusions: There is a paucity of empirical evidence for end-of-life symptom assessment and management for DCD patients. Key evidence gaps identified for DCD include the need for: i) a multidisciplinary structure of treatment teams and preferred environment for DCD, ii) objective tools for monitoring of distress in this patient population, and iii) evidence guiding the administration of analgesic medications following withdrawal of life sustaining therapy.
Keywords: donation after circulatory death; neurocritical care; organ donation; palliative care.
© The Author(s) 2022.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SPC has nothing to disclose, WSS reports Ownership interest in MindRhythm, Inc, RS has nothing to disclose, DBW reports grants as below during the conduct of the study; personal fees from American Thoracic Society, personal fees from UpToDate, outside the submitted work; SLM has nothing to disclose, CRF has nothing to disclose.
Figures
References
-
- Neyrinck A, Van Raemdonck D, Monbaliu D. Donation after circulatory death: current status. Curr Opin Anaesthesiol. 2013;1:382-390. - PubMed
-
- Smith M, Dominguez-Gil B, Greer DM, Manara AR, Souter MJ. Organ donation after circulatory death: current status and future potential. Intensive Care Med. 2019;45:310-321. - PubMed
-
- Institute of Medicine (US) . Committee on Non-Heart-Beating Transplantation II: The Scientific and Ethical Basis for Practice and Protocols. Non-Heart-Beating Organ Transplantation: Practice and Protocols. Washington (DC): National Academies Press (US); 2000. http://www.ncbi.nlm.nih.gov/books/NBK225025/ - PubMed
-
- Russell JA, Epstein LG, Greer DM, Kirschen M, Rubin MA, Lewis A. Brain death, the determination of brain death, and member guidance for brain death accommodation requests: AAN position statement. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. 2019;92:228-232. - PubMed
-
- A Position Paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine . Recommendations for nonheartbeating organ donation: Crit Care Med. 2001;29:1826–1831. - PubMed