Posttransplant de novo DSA and NDSA affect GvHD, OS, and DFS after haplo-HSCT in patients without pre-existing HLA antibodies of hematological malignancies
- PMID: 36532004
- PMCID: PMC9751004
- DOI: 10.3389/fimmu.2022.1047200
Posttransplant de novo DSA and NDSA affect GvHD, OS, and DFS after haplo-HSCT in patients without pre-existing HLA antibodies of hematological malignancies
Abstract
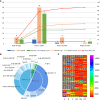
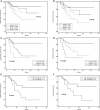
To examine the production time, type, and MFI of post-transplantation de novo HLA antibodies, and their effects on haplo-HSCT outcomes, we retrospectively included 116 patients who were negative for pre-existing HLA antibodies. In total, 322 serum samples from pre-transplantation to post-transplantation were dynamically tested by Luminex and single-antigen bead reagents. Patients were divided into: HLA antibody persistently negative group (group 1), the de novo HLA antibody transiently positive group (group 2), the de novo HLA antibody non-persistently positive group (group 3), and the de novo HLA antibody persistently positive group (group 4). Group 4 included DSA+non-DSA (NDSA) (group 4a) and NDSA (group 4b) groups. The detection rate of de novo HLA antibodies was 75.9% (88/116). The median MFI for de novo HLA antibodies was 2439 (1033-20162). The incidence of II-IV aGvHD was higher in group 2 than in group 1 (52.6% vs 17.9%, P < 0.01); in group 4a than in group 1 (87.5% vs 17.9%, P < 0.001); and in group 4a than in group 4b (87.5% vs 40.0%, P = 0.001). The DFS (37.5% vs 85.7%, P < 0.01) and OS (37.5% vs 85.7%, P < 0.01) of group 4a were lower than those of group 1. The DFS (48.0% vs 85.7%, P < 0.01) and OS (56.0% vs 85.7%, P = 0.03) of group 4b were lower than those of group 1. Multivariate analysis showed that de novo HLA antibody being transiently positive (HR: 5.30; 95% CI: 1.71-16.42, P = 0.01) and persistently positive (HR: 5.67; 95% CI: 2.00-16.08, P < 0.01) were both associated with a higher incidence of II-IV aGvHD. Persistently positive de novo HLA antibodies were a risk factor for reduced DFS (HR: 6.57; 95% CI: 2.08-20.70, P < 0.01) and OS (HR: 5.51; 95% CI: 1.73-17.53, P < 0.01). DSA and NDSA can be detected since 15 days after haplo-HSCT in patients without pre-existing HLA antibodies, and affect aGvHD, DFS, and OS. Haplo-HSCT patients must be monitored for HLA antibodies changes for appropriate preventive clinical management, and we recommend that 1-month post-transplantation is the best test time point.
Keywords: acute graft-versus-host disease (aGvHD); de novo human leukocyte antigen antibody (de novo HLA antibody); donor-specific anti-HLA antibody (DSA); haploidentical hematopoietic stem cell transplantation (haplo-HSCT); outcomes.
Copyright © 2022 Wang, Ji, Chen, Li, Zhu, Yuan, Bao, Wu and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
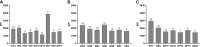

Similar articles
-
Clinical significance of low pre-transplant donor specific antibodies (DSA) in living donor kidney recipients with negative complement-dependent cytotoxicity crossmatches (CDCXM), and negative flow cytometry crossmatches (FLXM) - A single-center experience.Transpl Immunol. 2022 Oct;74:101672. doi: 10.1016/j.trim.2022.101672. Epub 2022 Jul 19. Transpl Immunol. 2022. PMID: 35868613
-
Outcome of Haploidentical Peripheral Blood Allografts Using Post-Transplantation Cyclophosphamide Compared to Matched Sibling and Unrelated Donor Bone Marrow Allografts in Pediatric Patients with Hematologic Malignancies: A Single-Center Analysis.Transplant Cell Ther. 2022 Mar;28(3):158.e1-158.e9. doi: 10.1016/j.jtct.2021.11.009. Epub 2021 Nov 25. Transplant Cell Ther. 2022. PMID: 34838785
-
[Effects of preexisting donor-specific HLA antibodies for graft failure in un-manipulated haploidentical hematopoietic stem cell transplantation].Zhonghua Xue Ye Xue Za Zhi. 2018 Mar 14;39(3):190-195. doi: 10.3760/cma.j.issn.0253-2727.2018.03.004. Zhonghua Xue Ye Xue Za Zhi. 2018. PMID: 29562462 Free PMC article. Chinese.
-
Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis.Stem Cell Res Ther. 2021 Apr 20;12(1):246. doi: 10.1186/s13287-021-02304-x. Stem Cell Res Ther. 2021. PMID: 33879242 Free PMC article.
-
Haploidentical stem cell transplantation vs matched unrelated donor transplantation in adults with hematologic malignancies: a systematic review and meta-analysis.Hematology. 2020 Dec;25(1):356-365. doi: 10.1080/16078454.2020.1831292. Hematology. 2020. PMID: 33054609
Cited by
-
The effects of intragraft CD38+ B cell on chronic active antibody mediated rejection in kidney transplantation.Int Urol Nephrol. 2025 Sep;57(9):2817-2828. doi: 10.1007/s11255-025-04451-z. Epub 2025 Mar 20. Int Urol Nephrol. 2025. PMID: 40111715
-
Desensitization Strategies for Donor-Specific Antibodies in HLA-Mismatched Stem Cell Transplantation Recipients: What We Know and What We Do Not Know.Oncol Ther. 2024 Sep;12(3):375-394. doi: 10.1007/s40487-024-00283-6. Epub 2024 Jun 15. Oncol Ther. 2024. PMID: 38879734 Free PMC article. Review.
References
-
- Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. . The European society for blood and marrow transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone marrow Transplant (2018) 53(5):521–34. doi: 10.1038/s41409-017-0062-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

