SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: Antibody-dependent as well as independent cell entry
- PMID: 36532011
- PMCID: PMC9751203
- DOI: 10.3389/fimmu.2022.1050478
SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: Antibody-dependent as well as independent cell entry
Abstract
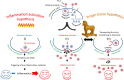
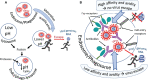
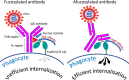
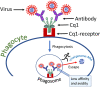
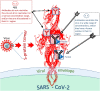
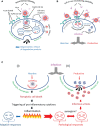
Our review summarizes the evidence that COVID-19 can be complicated by SARS-CoV-2 infection of immune cells. This evidence is widespread and accumulating at an increasing rate. Research teams from around the world, studying primary and established cell cultures, animal models, and analyzing autopsy material from COVID-19 deceased patients, are seeing the same thing, namely that some immune cells are infected or capable of being infected with the virus. Human cells most vulnerable to infection include both professional phagocytes, such as monocytes, macrophages, and dendritic cells, as well as nonprofessional phagocytes, such as B-cells. Convincing evidence has accumulated to suggest that the virus can infect monocytes and macrophages, while data on infection of dendritic cells and B-cells are still scarce. Viral infection of immune cells can occur directly through cell receptors, but it can also be mediated or enhanced by antibodies through the Fc gamma receptors of phagocytic cells. Antibody-dependent enhancement (ADE) most likely occurs during the primary encounter with the pathogen through the first COVID-19 infection rather than during the second encounter, which is characteristic of ADE caused by other viruses. Highly fucosylated antibodies of vaccinees seems to be incapable of causing ADE, whereas afucosylated antibodies of persons with acute primary infection or convalescents are capable. SARS-CoV-2 entry into immune cells can lead to an abortive infection followed by host cell pyroptosis, and a massive inflammatory cascade. This scenario has the most experimental evidence. Other scenarios are also possible, for which the evidence base is not yet as extensive, namely productive infection of immune cells or trans-infection of other non-immune permissive cells. The chance of a latent infection cannot be ruled out either.
Keywords: ADE; COVID-19 pathogenesis; SARS-CoV-2; immune cells infection; macrophage infection; monocyte infection; phagocyte infection; pyroptosis.
Copyright © 2022 Matveeva, Nechipurenko, Lagutkin, Yegorov and Kzhyshkowska.
Conflict of interest statement
Authors OM and DL was employed by Sendai Viralytics, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

