ATG-Fresenius increases the risk of red blood cell transfusion after kidney transplantation
- PMID: 36532030
- PMCID: PMC9753326
- DOI: 10.3389/fimmu.2022.1045580
ATG-Fresenius increases the risk of red blood cell transfusion after kidney transplantation
Abstract
Introduction: In sensitized deceased donor kidney allograft recipients, the most frequent induction therapy is anti-thymocyte globulins (ATG), including Thymoglobulin® (Thymo) and ATG-Fresenius (ATG-F).
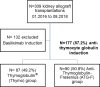
Methods: We conducted a 3-year monocentric observational study to compare the impact of ATGs on hematological parameters. We included adult kidney transplant recipients treated with ATG induction therapy, either Thymo or ATG-F, on a one-in-two basis. The primary endpoint was red blood cell (RBC) transfusions within 14 days after transplantation.
Results: Among 309 kidney allograft recipients, 177 (57.2%) received ATG induction, 90 (50.8 %) ATG-F, and 87 (49.2%) Thymo. The ATG-F group received significantly more RBC transfusions (63.3% vs. 46% p = 0.02) and in bigger volumes (p = 0.01). Platelet transfusion was similar in both groups. Within 14 and 30 days after transplantation, older age, ATG-F induction, and early surgical complication were independently associated with RBC transfusion. Patient survival rate was 95%, and the death-censored kidney allograft survival rate was 91.5% at 12 months post-transplantation. There was no difference in the incidence of acute rejection and infections or in the prevalence of anti-HLA donor-specific antibodies.
Discussion: In conclusion, after kidney transplantation, ATG-F is an independent risk factor for early RBC transfusion and early thrombocytopenia without clinical and biological consequences. These new data should be clinically considered, and alternatives to ATG should be further explored.
Keywords: anti-thymocyte globulins; donor-specific anti-HLA antibodies; induction therapy; kidney transplantation; red blood cell transfusion.
Copyright © 2022 Sebti, Petit-Hoang, Chami, Audureau, Cordonnier-Jourdin, Paul, Pourcine, Grimbert, Ourghanlian and Matignon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
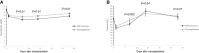
Similar articles
-
A Comparison of Two Types of Rabbit Antithymocyte Globulin Induction Therapy in Immunological High-Risk Kidney Recipients: A Prospective Randomized Control Study.PLoS One. 2016 Nov 17;11(11):e0165233. doi: 10.1371/journal.pone.0165233. eCollection 2016. PLoS One. 2016. PMID: 27855166 Free PMC article. Clinical Trial.
-
Comparison of efficacy and safety between rabbit anti-thymocyte globulin and anti-T lymphocyte globulin in kidney transplantation from donation after cardiac death: a retrospective cohort study.Nephrology (Carlton). 2015 Aug;20(8):539-43. doi: 10.1111/nep.12469. Nephrology (Carlton). 2015. PMID: 25808082
-
A retrospective comparison of the efficacy and safety in kidney transplant recipients with basiliximab and anti-thymocyte globulin.Chin Med J (Engl). 2012 Mar;125(6):1135-40. Chin Med J (Engl). 2012. PMID: 22613543
-
A review on comparing two commonly used rabbit anti-thymocyte globulins as induction therapy in solid organ transplantation.Expert Opin Biol Ther. 2013 Sep;13(9):1299-313. doi: 10.1517/14712598.2013.822064. Epub 2013 Jul 23. Expert Opin Biol Ther. 2013. PMID: 23875884 Review.
-
Anti-thymocyte globulin for treatment of T-cell-mediated allograft rejection.World J Transplant. 2023 Dec 18;13(6):299-308. doi: 10.5500/wjt.v13.i6.299. World J Transplant. 2023. PMID: 38174145 Free PMC article. Review.
Cited by
-
Comparison of ATG-thymoglobulin with atg-fresenius in patients with hematological malignancies who undergo allogeneic hematopoietic stem cell transplantation: a propensity score-matched analysis.Ann Hematol. 2025 Mar;104(3):1907-1916. doi: 10.1007/s00277-025-06267-4. Epub 2025 Feb 28. Ann Hematol. 2025. PMID: 40016396 Free PMC article.
References
-
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. . Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med (1999) 341:1725–30. doi: 10.1056/NEJM199912023412303 - DOI - PubMed
-
- Aikawa A. Current status and future aspects of kidney transplantation in Japan. Ren Replace Ther (2018) 4:50. doi: 10.1186/s41100-018-0186-3 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

