Impact of exposure to malaria and nutritional status on responses to the experimental malaria vaccine ChAd63 MVA ME-TRAP in 5-17 month-old children in Burkina Faso
- PMID: 36532031
- PMCID: PMC9755991
- DOI: 10.3389/fimmu.2022.1058227
Impact of exposure to malaria and nutritional status on responses to the experimental malaria vaccine ChAd63 MVA ME-TRAP in 5-17 month-old children in Burkina Faso
Abstract
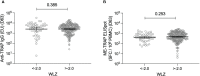
The experimental malaria vaccine ChAd63 MVA ME-TRAP previously showed protective efficacy against Plasmodium falciparum infection in Phase IIa sporozoite challenge studies in adults in the United Kingdom and in a Phase IIb field efficacy trial in Kenyan adults. However, it failed to demonstrate efficacy in a phase IIb trial in 5-17 month-old children in an area of high malaria transmission in Burkina Faso. This secondary analysis investigated whether exposure to malaria or nutritional status might be associated with reduced responses to vaccination in this cohort. Parasite blood smears and anti-AMA-1 IgG titres were used to assess history of exposure to malaria and weight-for-length Z scores were calculated to assess nutritional status. Differences in vaccine-specific anti-TRAP IgG titre and ex vivo IFNγ ELISpot response were measured between groups. In total, n = 336 volunteers randomised to receive the experimental vaccine regimen were included in this analysis. A positive smear microscopy result was associated with reduced anti-TRAP IgG titre (geometric mean titre: 2775 (uninfected) vs 1968 (infected), p = 0.025), whilst anti-AMA-1 IgG titres were weakly negatively correlated with reduced ex vivo IFNγ ELISpot response (r = -0.18, p = 0.008). Nutritional status was not associated with either humoral or cellular immunogenicity. Vaccine efficacy was also measured separately for vaccinees with positive and negative blood smears. Although not significant in either group compared to controls, vaccine efficacy measured by Cox hazard ratio was higher in uninfected compared to infected individuals (19.8% [p = 0.50] vs 3.3% [p = 0.69]). Overall, this data suggests exposure to malaria may be associated with impaired vaccine immunogenicity. This may have consequences for the testing and eventual deployment of various vaccines, in areas with high endemicity for malaria.
Trial registration: Pactr.org, identifier PACTR201208000404131; ClinicalTrials.gov, identifier NCT01635647.
Keywords: Burkina Faso; ME-TRAP; immunosuppression; malaria; nutrition; vaccine; viral vector.
Copyright © 2022 Morter, Tiono, Nébié, Hague, Ouedraogo, Diarra, Viebig, Hill, Ewer and Sirima.
Conflict of interest statement
AH is a named inventor on patent applications and issued patents relating to malaria vectored vaccines and immunization regimes. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5-17 months old infants and children.PLoS One. 2018 Dec 12;13(12):e0208328. doi: 10.1371/journal.pone.0208328. eCollection 2018. PLoS One. 2018. PMID: 30540808 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of heterologous prime-boost immunisation with Plasmodium falciparum malaria candidate vaccines, ChAd63 ME-TRAP and MVA ME-TRAP, in healthy Gambian and Kenyan adults.PLoS One. 2013;8(3):e57726. doi: 10.1371/journal.pone.0057726. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23526949 Free PMC article. Clinical Trial.
-
Safety, Immunogenicity and Efficacy of Prime-Boost Vaccination with ChAd63 and MVA Encoding ME-TRAP against Plasmodium falciparum Infection in Adults in Senegal.PLoS One. 2016 Dec 15;11(12):e0167951. doi: 10.1371/journal.pone.0167951. eCollection 2016. PLoS One. 2016. PMID: 27978537 Free PMC article. Clinical Trial.
-
Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals.J Infect Dis. 2015 Apr 1;211(7):1076-86. doi: 10.1093/infdis/jiu579. Epub 2014 Oct 21. J Infect Dis. 2015. PMID: 25336730 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of ChAd63 and MVA ME-TRAP in West African Children and Infants.Mol Ther. 2016 Aug;24(8):1470-7. doi: 10.1038/mt.2016.83. Epub 2016 Apr 25. Mol Ther. 2016. PMID: 27109630 Free PMC article. Clinical Trial.
Cited by
-
Plasmodium falciparum infection coinciding with the malaria vaccine candidate BK-SE36 administration interferes with the immune responses in Burkinabe children.Front Immunol. 2023 Mar 10;14:1119820. doi: 10.3389/fimmu.2023.1119820. eCollection 2023. Front Immunol. 2023. PMID: 36993981 Free PMC article.
-
Previous Malaria Exposures and Immune Dysregulation: Developing Strategies To Improve Malaria Vaccine Efficacy in Young Children.Am J Trop Med Hyg. 2024 Mar 5;110(4):627-630. doi: 10.4269/ajtmh.23-0696. Print 2024 Apr 3. Am J Trop Med Hyg. 2024. PMID: 38442424 Free PMC article.
-
Immunological factors linked to geographical variation in vaccine responses.Nat Rev Immunol. 2024 Apr;24(4):250-263. doi: 10.1038/s41577-023-00941-2. Epub 2023 Sep 28. Nat Rev Immunol. 2024. PMID: 37770632 Review.
-
Evaluating artesunate monotherapy and dihydroartemisinin-piperaquine as potential antimalarial options for prevaccination radical cures during future malaria vaccine field efficacy trials.Malar J. 2024 Dec 18;23(1):377. doi: 10.1186/s12936-024-05198-1. Malar J. 2024. PMID: 39695728 Free PMC article. Clinical Trial.
-
Role of homologous recombination/recombineering on human adenovirus genome engineering: Not the only but the most competent solution.Eng Microbiol. 2024 Feb 8;4(1):100140. doi: 10.1016/j.engmic.2024.100140. eCollection 2024 Mar. Eng Microbiol. 2024. PMID: 39628785 Free PMC article. Review.
References
-
- World Health Organisation . World malaria report 2021 (2021). Available at: https://www.who.int/publications/i/item/9789240040496 (Accessed January 4, 2022).
-
- WHO recommends groundbreaking malaria vaccine for children at risk . Available at: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-m... (Accessed January 4, 2022).
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

