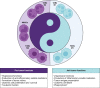
The Yin-Yang of myeloid cells in the leukemic microenvironment: Immunological role and clinical implications
- PMID: 36532078
- PMCID: PMC9751477
- DOI: 10.3389/fimmu.2022.1071188
The Yin-Yang of myeloid cells in the leukemic microenvironment: Immunological role and clinical implications
Abstract
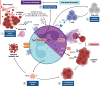
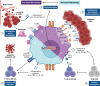
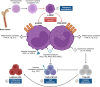
The leukemic microenvironment has a high diversity of immune cells that are phenotypically and functionally distinct. However, our understanding of the biology, immunology, and clinical implications underlying these cells remains poorly investigated. Among the resident immune cells that can infiltrate the leukemic microenvironment are myeloid cells, which correspond to a heterogeneous cell group of the innate immune system. They encompass populations of neutrophils, macrophages, and myeloid-derived suppressor cells (MDSCs). These cells can be abundant in different tissues and, in the leukemic microenvironment, are associated with the clinical outcome of the patient, acting dichotomously to contribute to leukemic progression or stimulate antitumor immune responses. In this review, we detail the current evidence and the many mechanisms that indicate that the activation of different myeloid cell populations may contribute to immunosuppression, survival, or metastatic dissemination, as well as in immunosurveillance and stimulation of specific cytotoxic responses. Furthermore, we broadly discuss the interactions of tumor-associated neutrophils and macrophages (TANs and TAMs, respectively) and MDSCs in the leukemic microenvironment. Finally, we provide new perspectives on the potential of myeloid cell subpopulations as predictive biomarkers of therapeutical response, as well as potential targets in the chemoimmunotherapy of leukemias due to their dual Yin-Yang roles in leukemia.
Keywords: immune response; immunotherapy; leukemia; macrophages; myeloid-derived suppressor cells; neutrophils; tumor microenvironment.
Copyright © 2022 Magalhães-Gama, Alves-Hanna, Araújo, Barros, Silva, Catão, Moraes, Freitas, Tarragô, Malheiro, Teixeira-Carvalho and Costa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Diamonds in the Rough: Harnessing Tumor-Associated Myeloid Cells for Cancer Therapy.Front Immunol. 2018 Oct 8;9:2250. doi: 10.3389/fimmu.2018.02250. eCollection 2018. Front Immunol. 2018. PMID: 30349530 Free PMC article. Review.
-
Myeloid suppressor cells in cancer and autoimmunity.J Autoimmun. 2017 Dec;85:117-125. doi: 10.1016/j.jaut.2017.07.010. Epub 2017 Jul 17. J Autoimmun. 2017. PMID: 28728794 Review.
-
Harnessing myeloid cells in cancer.Mol Cancer. 2025 Mar 6;24(1):69. doi: 10.1186/s12943-025-02249-2. Mol Cancer. 2025. PMID: 40050933 Free PMC article. Review.
-
Tumor-infiltrating myeloid cells; mechanisms, functional significance, and targeting in cancer therapy.Cell Oncol (Dordr). 2025 Jun;48(3):559-590. doi: 10.1007/s13402-025-01051-y. Epub 2025 Feb 25. Cell Oncol (Dordr). 2025. PMID: 39998754 Free PMC article. Review.
-
Yin-yang effect of tumor infiltrating B cells in breast cancer: From mechanism to immunotherapy.Cancer Lett. 2017 May 1;393:1-7. doi: 10.1016/j.canlet.2017.02.008. Epub 2017 Feb 16. Cancer Lett. 2017. PMID: 28216374 Review.
Cited by
-
Insights Regarding the Role of Inflammasomes in Leukemia: What Do We Know?J Immunol Res. 2023 Aug 4;2023:5584492. doi: 10.1155/2023/5584492. eCollection 2023. J Immunol Res. 2023. PMID: 37577033 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

