Autologous bone marrow-derived MSCs engineered to express oFVIII-FLAG engraft in adult sheep and produce an effective increase in plasma FVIII levels
- PMID: 36532079
- PMCID: PMC9755880
- DOI: 10.3389/fimmu.2022.1070476
Autologous bone marrow-derived MSCs engineered to express oFVIII-FLAG engraft in adult sheep and produce an effective increase in plasma FVIII levels
Abstract
Introduction: Hemophilia A (HA) is the most common X-linked bleeding disorder, occurring in 1 in 5,000 live male births and affecting >1 million individuals worldwide. Although advances in protein-based HA therapeutics have improved health outcomes, current standard-of-care requires infusion 2-3 times per week for life, and 30% of patients develop inhibitors, significantly increasing morbidity and mortality. There are thus unmet medical needs requiring novel approaches to treat HA.
Methods: We tested, in a highly translational large animal (sheep) model, whether the unique immunological and biological properties of autologous bone marrow (BM)-derived mesenchymal stromal cells (MSCs) could enable them to serve as cellular delivery vehicles to provide long-term expression of FVIII, avoiding the need for frequent infusions.
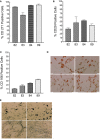
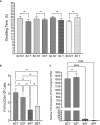
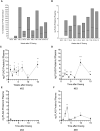
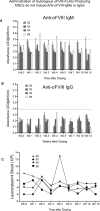
Results: We show that autologous BM-MSCs can be isolated, transduced with a lentivector to produce high levels of ovine (o)FVIII, extensively expanded, and transplanted into adult animals safely. The transplanted cells engraft in multiple organs, and they stably produce and secrete sufficient quantities of FVIII to yield elevated plasma FVIII levels for at least 15 weeks.
Discussion: These studies thus highlight the promise of cellular-based gene delivery approaches for treating HA.
Keywords: FVIII; Hemophilia A; bone marrow; cell therapy; efficacy & safety; gene therapy; mesenchymal stroma cell.
Copyright © 2022 Trevisan, Rodriguez, Medder, Lankford, Combs, Owen, Atala, Porada and Almeida-Porada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
In Utero Transplantation of Placenta-Derived Mesenchymal Stromal Cells for Potential Fetal Treatment of Hemophilia A.Cell Transplant. 2018 Jan;27(1):130-139. doi: 10.1177/0963689717728937. Cell Transplant. 2018. PMID: 29562772 Free PMC article.
-
Transplanting FVIII/ET3-secreting cells in fetal sheep increases FVIII levels long-term without inducing immunity or toxicity.Nat Commun. 2023 Jul 14;14(1):4206. doi: 10.1038/s41467-023-39986-1. Nat Commun. 2023. PMID: 37452013 Free PMC article.
-
Bone marrow stromal cells as targets for gene therapy of hemophilia A.Hum Gene Ther. 1998 Feb 10;9(3):353-65. doi: 10.1089/hum.1998.9.3-353. Hum Gene Ther. 1998. PMID: 9508053
-
Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A.Curr Gene Ther. 2003 Feb;3(1):27-41. doi: 10.2174/1566523033347417. Curr Gene Ther. 2003. PMID: 12553533 Review.
-
Coagulation factors with improved properties for hemophilia gene therapy.Semin Thromb Hemost. 2004 Apr;30(2):227-37. doi: 10.1055/s-2004-825636. Semin Thromb Hemost. 2004. PMID: 15118934 Review.
Cited by
-
Hemophilia A: An Ideal Disease for Prenatal Therapy.Prenat Diagn. 2025 Jun 10:10.1002/pd.6833. doi: 10.1002/pd.6833. Online ahead of print. Prenat Diagn. 2025. PMID: 40495292 Review.
References
-
- Joswig A-J, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R, et al. . Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther (2017) 8(1):42. doi: 10.1186/s13287-017-0503-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

