Liquid chromatography-tandem mass spectrometry for clinical diagnostics
- PMID: 36532107
- PMCID: PMC9735147
- DOI: 10.1038/s43586-022-00175-x
Liquid chromatography-tandem mass spectrometry for clinical diagnostics
Abstract
Mass spectrometry is a powerful analytical tool used for the analysis of a wide range of substances and matrices; it is increasingly utilized for clinical applications in laboratory medicine. This Primer includes an overview of basic mass spectrometry concepts, focusing primarily on tandem mass spectrometry. We discuss experimental considerations and quality management, and provide an overview of some key applications in the clinic. Lastly, the Primer discusses significant challenges for implementation of mass spectrometry in clinical laboratories and provides an outlook of where there are emerging clinical applications for this technology.
Keywords: Mass spectrometry; Medical research.
© Springer Nature Limited 2022, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsW.A.C. declares consulting for and research support from Thermo Fisher Scientific, consulting for Roche Diagnostics, and research support from Shimadzu Scientific Instruments. The other authors declare no competing interests.
Figures


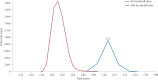
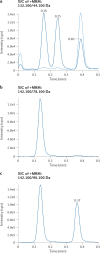
References
-
- Hage, D.S. in Contemporary Practice in Clinical Chemistry 4th edn Ch. 8 (eds Marzinke, M. A. & Clarke, W.) 135–157 (Elsevier, 2020).
-
- CLSI. Liquid Chromatography–Mass Spectrometry Methods; Approved Guideline CLSI Document C62-A (Clinical and Laboratory Standards Institute, 2014).
