Mechanistic insights into the pleiotropic effects of butyrate as a potential therapeutic agent on NAFLD management: A systematic review
- PMID: 36532559
- PMCID: PMC9755748
- DOI: 10.3389/fnut.2022.1037696
Mechanistic insights into the pleiotropic effects of butyrate as a potential therapeutic agent on NAFLD management: A systematic review
Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic diseases worldwide. As a multifaceted disease, NAFLD's pathogenesis is not entirely understood, but recent evidence reveals that gut microbiota plays a significant role in its progression. Butyrate, a gut microbiota metabolite, has been reported to have hepato-protective effects in NAFLD animal models. The purpose of this systematic review is to determine how butyrate affects the risk factors for NAFLD. Searches were conducted using relevant keywords in electronic databases up to March 2022. According to the evidence presented in this study, butyrate contributes to a wide variety of biological processes in the gut-liver axis. Its beneficial properties include improving intestinal homeostasis and liver health as well as anti-inflammatory, metabolism regulatory and anti-oxidative effects. These effects may be attributed to butyrate's ability to regulate gene expression as an epigenetic modulator and trigger cellular responses as a signalling molecule. However, the exact underlying mechanisms remain unclear. Human trials have not been performed on the effect of butyrate on NAFLD, so there are concerns about whether the results of animal studies can be translated to humans. This review summarises the current knowledge about the properties of butyrate, particularly its potential effects and mechanisms on liver health and NAFLD management.
Keywords: NAFLD; butyrate; gut microbiota; insulin resistance; obesity.
Copyright © 2022 Amiri, Arefhosseini, Bakhshimoghaddam, Jamshidi Gurvan and Hosseini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
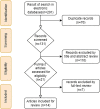
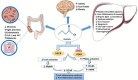
References
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. (2012) 142:1592–609. 10.1053/j.gastro.2012.04.001 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

