Antimelanogenesis Effect of Methyl Gallate through the Regulation of PI3K/Akt and MEK/ERK in B16F10 Melanoma Cells
- PMID: 36532851
- PMCID: PMC9750762
- DOI: 10.1155/2022/5092655
Antimelanogenesis Effect of Methyl Gallate through the Regulation of PI3K/Akt and MEK/ERK in B16F10 Melanoma Cells
Abstract
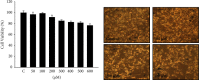
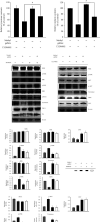
Methyl gallate is a polyphenolic compound found in many plants, and its antioxidant, antitumor, antibacterial, and anti-inflammatory effects have been extensively studied. More recently, antidepressant-like effects of methyl gallate have been demonstrated in some studies. In the present study, we examined the effects of methyl gallate on melanogenesis, including the tyrosinase inhibitory effect, the melanin content, and the molecular signaling pathways involved in this inhibition. The results showed that methyl gallate inhibited tyrosinase activity and significantly downregulated the expressions of melanin synthesis-associated proteins, including microphthalmia-associated transcription factor (MITF), tyrosinase, dopachrome tautomerase (Dct), and tyrosinase-related protein-1 (TRP1). In conclusion, our findings indicated that activation of MEK/ERK and PI3K/Akt promoted by methyl gallate caused downregulation of MITF and triggered its downstream signaling pathway, thereby inhibiting the production of melanin. In summary, methyl gallate showed significant inhibitory activity against melanin formation, implying that it may be a potential ingredient for application in skin-whitening cosmetics.
Copyright © 2022 Zhi Jiao Cheng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Inhibition of melanogenesis by gallic acid: possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells.Int J Mol Sci. 2013 Oct 14;14(10):20443-58. doi: 10.3390/ijms141020443. Int J Mol Sci. 2013. PMID: 24129178 Free PMC article.
-
Partially purified components of Nardostachys chinensis suppress melanin synthesis through ERK and Akt signaling pathway with cAMP down-regulation in B16F10 cells.J Ethnopharmacol. 2011 Oct 11;137(3):1207-14. doi: 10.1016/j.jep.2011.07.047. Epub 2011 Jul 26. J Ethnopharmacol. 2011. PMID: 21816215
-
Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways.J Ethnopharmacol. 2014;151(1):386-93. doi: 10.1016/j.jep.2013.10.054. Epub 2013 Nov 7. J Ethnopharmacol. 2014. PMID: 24212072
-
Betulinic acid isolated from Vitis amurensis root inhibits 3-isobutyl-1-methylxanthine induced melanogenesis via the regulation of MEK/ERK and PI3K/Akt pathways in B16F10 cells.Food Chem Toxicol. 2014 Jun;68:38-43. doi: 10.1016/j.fct.2014.03.001. Epub 2014 Mar 12. Food Chem Toxicol. 2014. PMID: 24632067
-
Molecular understanding of the therapeutic potential of melanin inhibiting natural products.RSC Med Chem. 2024 May 10;15(7):2226-2253. doi: 10.1039/d4md00224e. eCollection 2024 Jul 17. RSC Med Chem. 2024. PMID: 39026645 Free PMC article. Review.
Cited by
-
Inhibitory effects of corylin derived from aerial part of Pueraria lobata on melanin synthesis and potential applications in skin whitening and photoaging management.Nat Prod Bioprospect. 2025 Apr 27;15(1):27. doi: 10.1007/s13659-025-00509-8. Nat Prod Bioprospect. 2025. PMID: 40287902 Free PMC article.
-
Syringetin Promotes Melanogenesis in B16F10 Cells.Int J Mol Sci. 2023 Jun 9;24(12):9960. doi: 10.3390/ijms24129960. Int J Mol Sci. 2023. PMID: 37373110 Free PMC article.
-
Multifunctional cosmetic potential of extracellular vesicle‑like nanoparticles derived from the stem of Cannabis sativa in treating pigmentation disorders.Mol Med Rep. 2025 Jun;31(6):147. doi: 10.3892/mmr.2025.13512. Epub 2025 Apr 4. Mol Med Rep. 2025. PMID: 40183388 Free PMC article.
-
Inhibitory Effects of Polyphenol- and Flavonoid-Enriched Rice Seed Extract on Melanogenesis in Melan-a Cells via MAPK Signaling-Mediated MITF Downregulation.Int J Mol Sci. 2023 Jul 24;24(14):11841. doi: 10.3390/ijms241411841. Int J Mol Sci. 2023. PMID: 37511600 Free PMC article.
-
Acenocoumarol, an Anticoagulant Drug, Prevents Melanogenesis in B16F10 Melanoma Cells.Pharmaceuticals (Basel). 2023 Apr 17;16(4):604. doi: 10.3390/ph16040604. Pharmaceuticals (Basel). 2023. PMID: 37111361 Free PMC article.
References
-
- Rojekar M. V., Sawant S. A short review of pigmentation disorders in systemic diseases. Pigmentary Disorders . 2015;02(04) doi: 10.4172/2376-0427.1000174. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

