Quasicriticality explains variability of human neural dynamics across life span
- PMID: 36532868
- PMCID: PMC9747757
- DOI: 10.3389/fncom.2022.1037550
Quasicriticality explains variability of human neural dynamics across life span
Abstract
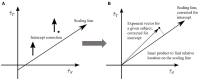
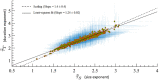
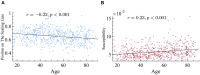
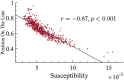
Aging impacts the brain's structural and functional organization and over time leads to various disorders, such as Alzheimer's disease and cognitive impairment. The process also impacts sensory function, bringing about a general slowing in various perceptual and cognitive functions. Here, we analyze the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) resting-state magnetoencephalography (MEG) dataset-the largest aging cohort available-in light of the quasicriticality framework, a novel organizing principle for brain functionality which relates information processing and scaling properties of brain activity to brain connectivity and stimulus. Examination of the data using this framework reveals interesting correlations with age and gender of test subjects. Using simulated data as verification, our results suggest a link between changes to brain connectivity due to aging and increased dynamical fluctuations of neuronal firing rates. Our findings suggest a platform to develop biomarkers of neurological health.
Keywords: Cam-CAN dataset; aging; healthy human; magnetoencephalography (MEG); neuronal avalanches; quasicriticality.
Copyright © 2022 Fosque, Alipour, Zare, Williams-García, Beggs and Ortiz.
Conflict of interest statement
Author MZ was employed by CGI. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



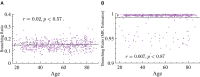
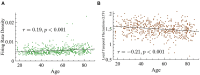


Similar articles
-
Near-Critical Dynamics in Stimulus-Evoked Activity of the Human Brain and Its Relation to Spontaneous Resting-State Activity.J Neurosci. 2015 Oct 14;35(41):13927-42. doi: 10.1523/JNEUROSCI.0477-15.2015. J Neurosci. 2015. PMID: 26468194 Free PMC article.
-
Characterizing the fluctuations of dynamic resting-state electrophysiological functional connectivity: reduced neuronal coupling variability in mild cognitive impairment and dementia due to Alzheimer's disease.J Neural Eng. 2019 Sep 17;16(5):056030. doi: 10.1088/1741-2552/ab234b. J Neural Eng. 2019. PMID: 31112938
-
The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample.Neuroimage. 2017 Jan;144(Pt B):262-269. doi: 10.1016/j.neuroimage.2015.09.018. Epub 2015 Sep 12. Neuroimage. 2017. PMID: 26375206 Free PMC article.
-
Functional brain connectivity using fMRI in aging and Alzheimer's disease.Neuropsychol Rev. 2014 Mar;24(1):49-62. doi: 10.1007/s11065-014-9249-6. Epub 2014 Feb 23. Neuropsychol Rev. 2014. PMID: 24562737 Free PMC article. Review.
-
Healthy and Pathological Brain Aging: From the Perspective of Oscillations, Functional Connectivity, and Signal Complexity.Neuropsychobiology. 2017;75(4):151-161. doi: 10.1159/000486870. Epub 2018 Feb 21. Neuropsychobiology. 2017. PMID: 29466802 Review.
Cited by
-
Extreme signal amplitude events in neuromagnetic oscillations reveal brain aging processing across adulthood.Front Aging Neurosci. 2025 Mar 4;17:1498400. doi: 10.3389/fnagi.2025.1498400. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40103930 Free PMC article.
-
Critical-like Brain Dynamics in a Continuum from Second- to First-Order Phase Transition.J Neurosci. 2023 Nov 8;43(45):7642-7656. doi: 10.1523/JNEUROSCI.1889-22.2023. Epub 2023 Oct 10. J Neurosci. 2023. PMID: 37816599 Free PMC article.
-
Failure in a population: Tauopathy disrupts homeostatic set-points in emergent dynamics despite stability in the constituent neurons.Neuron. 2024 Nov 6;112(21):3567-3584.e5. doi: 10.1016/j.neuron.2024.08.006. Epub 2024 Sep 5. Neuron. 2024. PMID: 39241778
-
Tauopathy severely disrupts homeostatic set-points in emergent neural dynamics but not in the activity of individual neurons.bioRxiv [Preprint]. 2023 Sep 6:2023.09.01.555947. doi: 10.1101/2023.09.01.555947. bioRxiv. 2023. PMID: 37732214 Free PMC article. Preprint.
-
Mapping and modeling age-related changes in intrinsic neural timescales.Commun Biol. 2025 Feb 3;8(1):167. doi: 10.1038/s42003-025-07517-x. Commun Biol. 2025. PMID: 39901043 Free PMC article.
References
-
- Beggs J. (2022). The Cortex and the Critical Point: Understanding the Power of Emergence. Cambridge, MA: MIT Press. 10.7551/mitpress/13588.001.0001 - DOI
LinkOut - more resources
Full Text Sources

