Efficacy and Safety of Molnupiravir in Mild COVID-19 Patients in India
- PMID: 36532902
- PMCID: PMC9751243
- DOI: 10.7759/cureus.31508
Efficacy and Safety of Molnupiravir in Mild COVID-19 Patients in India
Abstract
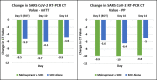
Background At the peak of the coronavirus disease 2019 (COVID-19) pandemic, the need for an orally administered agent to prevent the progression of acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection became increasingly evident, which was the impetus behind our investigations with molnupiravir. Molnupiravir has been shown to be effective in preventing hospitalizations and/or clinical complications in patients with mild-to-moderate COVID-19. In this study, we evaluate the efficacy and safety of molnupiravir in Indian patients with mild SARS-CoV-2 infection and at least one risk factor for disease progression (CTRI/2021/05/033739). Methodology This was a phase III, multicenter, randomized, open-label, controlled study conducted in Indian adults aged 18-60 years with mild SARS-CoV-2, reverse transcription polymerase chain reaction (RT-PCR)-positive within 48 hours of enrollment in the study, and within five days of first symptom onset. Enrolled patients were randomized to treatment arms in a 1:1 ratio to receive molnupiravir or placebo in addition to the standard of care (SoC) for SARS-CoV-2 infection. The SoC was in compliance with Government of India guidelines that were in force at the time. The primary endpoint was the rate of hospitalization up to day 14. Safety endpoints included incidence of adverse events (AEs). Results Eligible patients were randomized in a 1:1 ratio to receive molnupiravir in addition to SoC treatment (n = 608) or SoC alone (n = 610). In the molnupiravir group, nine (1.48%) patients required hospitalization versus 26 (4.26%) patients in the control group (risk difference = -2.78%; 95% CI = -4.65, -0.90; p = 0.0053). Overall, 45 (3.70%) patients reported 47 AEs during the study, most of which were mild and resolved completely. The molnupiravir group reported 30 AEs compared to 17 AEs in the control group. Headache and nausea were the two most commonly reported AEs. Conclusions The molnupiravir arm showed a lower rate of hospitalization and a shorter time for the improvement of clinical symptoms coupled with early RT-PCR negativity. Molnupiravir was well tolerated, and AEs were mild and rare. The addition of molnupiravir to standard therapy has the potential to prevent the progression of mild COVID-19 disease to the severe form.
Keywords: efficacy and safety; molnupiravir; oral antiviral drug; phase 3 study; sars-cov-2 infection.
Copyright © 2022, Sinha et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- FDA approves first treatment for COVID-19. [ Sep; 2022 ]. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-t... https://www.fda.gov/news-events/press-announcements/fda-approves-first-t...
-
- COVID-19 treatment: investigational drugs and other therapies. [ Sep; 2022 ]. 2022. https://emedicine.medscape.com/article/2500116-overview https://emedicine.medscape.com/article/2500116-overview
-
- FDA halts use of COVID drugs ineffective against omicron. [ Sep; 2022 ]. 2022. https://www.webmd.com/lung/news/20220125/fda_halts_covid_drugs https://www.webmd.com/lung/news/20220125/fda_halts_covid_drugs
-
- Coronavirus (COVID-19) update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant. [ Sep; 2022 ];https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... 2022 on:22–27.
-
- A concise route to MK-4482 (EIDD-2801) from cytidine. Vasudevan N, Ahlqvist GP, McGeough CP, et al. Chem Commun (Camb) 2020;56:13363–13364. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous