2-hydroxyisobutyric acid (2-HIBA) modulates ageing and fat deposition in Caenorhabditis elegans
- PMID: 36533081
- PMCID: PMC9749906
- DOI: 10.3389/fmolb.2022.986022
2-hydroxyisobutyric acid (2-HIBA) modulates ageing and fat deposition in Caenorhabditis elegans
Abstract
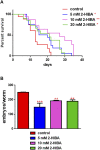
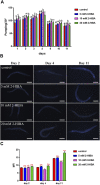
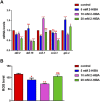
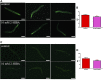
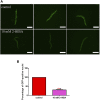
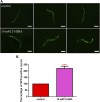
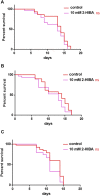
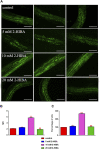
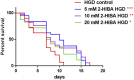
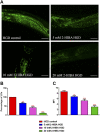
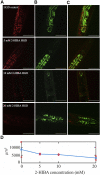
High levels of 2-hydroxyisobutyric acid (2-HIBA) were found in urines of patients with obesity and hepatic steatosis, suggesting a potential involvement of this metabolite in clinical conditions. The gut microbial origin of 2-HIBA was hypothesized, however its actual origin and role in biological processes are still not clear. We investigated how treatment with 2-HIBA affected the physiology of the model organism Caenorhabditis elegans, in both standard and high-glucose diet (HGD) growth conditions, by targeted transcriptomic and metabolomic analyses, Coherent Anti-Stokes Raman Scattering (CARS) and two-photon fluorescence microscopy. In standard conditions, 2-HIBA resulted particularly effective to extend the lifespan, delay ageing processes and stimulate the oxidative stress resistance in wild type nematodes through the activation of insulin/IGF-1 signaling (IIS) and p38 MAPK pathways and, consequently, through a reduction of ROS levels. Moreover, variations of lipid accumulation observed in treated worms correlated with transcriptional levels of fatty acid synthesis genes and with the involvement of peptide transporter PEP-2. In HGD conditions, the effect of 2-HIBA on C. elegans resulted in a reduction of the lipid droplets deposition, accordingly with an increase of acs-2 gene transcription, involved in β-oxidation processes. In addition, the pro-longevity effect appeared to be correlated to higher levels of tryptophan, which may play a role in restoring the decreased viability observed in the HGD untreated nematodes.
Keywords: 2-hydroxyisobutyric acid; CARS; Caenorhabditis elegans; ageing; high-glucose diet; lipid metabolism; metabolomics; oxidative stress.
Copyright © 2022 Schifano, Conta, Preziosi, Ferrante, Batignani, Mancini, Tomassini, Sciubba, Scopigno, Uccelletti and Miccheli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alcántar-Fernández J., González-Maciel A., Reynoso-Robles R., Pérez Andrade M. E., Hernández-Vázquez A. de J., Velázquez-Arellano A., et al. (2019). High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans . PLoS ONE 14, e0226652. 10.1371/journal.pone.0226652 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

