Circ-Vps41 positively modulates Syp and its overexpression improves memory ability in aging mice
- PMID: 36533129
- PMCID: PMC9756809
- DOI: 10.3389/fnmol.2022.1037912
Circ-Vps41 positively modulates Syp and its overexpression improves memory ability in aging mice
Abstract
Introduction: Age is an established risk factor for neurodegenerative disorders. Aging-related cognitive decline is a common cause of memory impairment in aging individuals, in which hippocampal synaptic plasticity and hippocampus-dependent memory formation are damaged. Circular RNAs (circRNAs) have been reported in many cognitive disorders, but their role in aging-related memory impairment is unclear.Methods: In this study, we aimed to investigate the effects of circ-Vps41 on aging-related hippocampus-dependent memory impairment and explore the potential mechanisms. Here, D-galactose was used to produce a conventional aging model resulting in memory dysfunction.
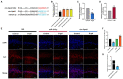
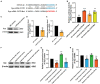
Results: Circ-Vps41 was significantly downregulated in D-galactose-induced aging in vitro and in vivo. The overexpression of circ-Vps41 could upregulate synaptophysin (Syp), thereby promoting the synaptic plasticity and alleviating cognitive impairment in aging mice. Mechanistically, we found that circ-Vps41 upregulated Syp expression by physically binding to miR-24-3p. Moreover, the miR-24-3p mimics reversed the circ-Vps41 overexpression-induced increase in Syp expression.
Discussion: Overexpression of circ-Vps41 alleviated the synaptic plasticity and memory dysfunction via the miR-24-3p/Syp axis. These findings revealed circ-Vps41 regulatory network and provided new insights into its potential mechanisms for improving aging-related learning and memory impairment.
Keywords: aging; circ-Vps41; learning and memory; miR-24a-3p; synaptophysin.
Copyright © 2022 Li, Wang, Gao, Zhang, Liu, Xie, Liu, Geng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

