Raising the standards of patient-centered outcomes research in myelodysplastic syndromes: Clinical utility and validation of the subscales of the QUALMS from the MDS-RIGHT project
- PMID: 36533415
- PMCID: PMC10067097
- DOI: 10.1002/cam4.5487
Raising the standards of patient-centered outcomes research in myelodysplastic syndromes: Clinical utility and validation of the subscales of the QUALMS from the MDS-RIGHT project
Abstract
Background: Clinical decision-making for patients with myelodysplastic syndromes (MDS) is challenging, and both disease and treatment effects heavily impact health-related quality of life (HRQoL) of these patients. Therefore, disease-specific HRQoL measures can be critical to harness the patient voice in MDS research.
Methods: We report a prospective international validation study of the Quality of Life in Myelodysplasia Scale (QUALMS) with a main focus on providing information on the psychometric characteristics of its three subscales: physical burden (QUALMS-P), emotional burden (QUALMS-E), and benefit finding (QUALMS-BF). The analysis is based on patients enrolled from three European countries and Israel, participating to the MDS-RIGHT Project. The scale structure and psychometric properties of the QUALMS were assessed.
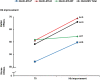
Results: Overall, 270 patients with a median age of 74 years were analyzed and the majority of them (60.3%) had a low MDS-Comorbidity Index score. Results of the confirmatory factor analysis supported the underlying scale structure of the QUALMS, which, in addition to a total score, includes three subscales: QUALMS-P, QUALMS-E, and the QUALMS-BF. The QUALMS-P exhibited the highest Cronbach's alpha coefficients. Discriminant validity analysis indicated good results with the QUALMS-P and QUALMS-E distinguishing between patients with different performance status, comorbidity, anemia, and transfusion dependency status. No floor and ceiling effects were observed. Responsiveness to change analysis supported the validity of the measure. Patients with a hemoglobin (Hb) level of <11 g/dL at study entry, who subsequently showed an improvement in their Hb levels, also reported a mean score change of 9 and 8 points (scales ranging between 0 and 100) in the expected direction of the QUALMS-E and QUALMS-P, respectively.
Conclusions: Our study provides additional validation data on the QUALMS from the international MDS-RIGHT Project. The use of this disease-specific HRQoL measure may contribute to raise quality standards of patient-centered outcomes research in MDS.
Keywords: myelodysplasia; myelodysplastic syndromes; patient-reported outcomes; quality of life; questionnaire; symptom burden.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Fabio Efficace: personal fees from Amgen, Abbvie, Janssen, and Novartis. Research support (to Institution) from Abbvie, Amgen, Novartis, all unrelated to this work. Corine van Marrewijk: project manager of the EUMDS Registry, is funded from the EUMDS (educational grants from Novartis Pharmacy B.V. Oncology Europe, Amgen Limited, Celgene International, Janssen Pharmaceutica, and Takeda Pharmaceuticals International) and MDS‐RIGHT (grant from EU's Horizon 2020 programme) project budgets. Moshe Mittelman: research grant: Janssen, Roche, Novartis, Medison/Amgen, Celgene/BMS, Abbvie, Gilead. Clinical trials sponsored by: Novartis, Takeda, Fibrogen, Celgene/BMS, Geron. Advisory board: Onconova, Novartis, Takeda, Silence, Astellas. Consulting fees: MDS HUB. Speakers' bureau: Celgene/BMS, Novartis, Media Digital. Reinhard Stauder: Celgene, BMS, Novartis (Advisory board); Celgene/BMS, Novartis (Honoraria); Celgene and BMS (Research funding), all unrelated to this work. Theo de Witte: Research Funding: Amgen, Celgene, Janssen, Novartis, Takeda.
Figures

References
-
- Platzbecker U. Treatment of MDS. Blood. 2019;133(10):1096‐1107. - PubMed
-
- Steensma DP, Heptinstall KV, Johnson VM, et al. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): results of a large internet‐based survey. Leuk Res. 2008;32(5):691‐698. - PubMed
-
- Efficace F, Gaidano G, Breccia M, et al. Prevalence, severity and correlates of fatigue in newly diagnosed patients with myelodysplastic syndromes. Br J Haematol. 2015;168(3):361‐370. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

