HP1 proteins regulate nucleolar structure and function by secluding pericentromeric constitutive heterochromatin
- PMID: 36533441
- PMCID: PMC9841413
- DOI: 10.1093/nar/gkac1159
HP1 proteins regulate nucleolar structure and function by secluding pericentromeric constitutive heterochromatin
Abstract
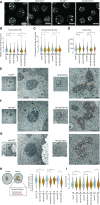
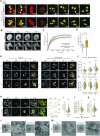
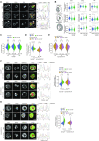
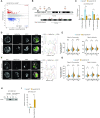
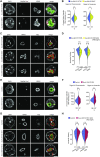
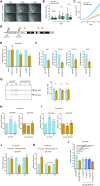
Nucleoli are nuclear compartments regulating ribosome biogenesis and cell growth. In embryonic stem cells (ESCs), nucleoli containing transcriptionally active ribosomal genes are spatially separated from pericentromeric satellite repeat sequences packaged in largely repressed constitutive heterochromatin (PCH). To date, mechanisms underlying such nuclear partitioning and the physiological relevance thereof are unknown. Here we show that repressive chromatin at PCH ensures structural integrity and function of nucleoli during cell cycle progression. Loss of heterochromatin proteins HP1α and HP1β causes deformation of PCH, with reduced H3K9 trimethylation (H3K9me3) and HP1γ levels, absence of H4K20me3 and upregulated major satellites expression. Spatially, derepressed PCH aberrantly associates with nucleoli accumulating severe morphological defects during S/G2 cell cycle progression. Hp1α/β deficiency reduces cell proliferation, ribosomal RNA biosynthesis and mobility of Nucleophosmin, a major nucleolar component. Nucleolar integrity and function require HP1α/β proteins to be recruited to H3K9me3-marked PCH and their ability to dimerize. Correspondingly, ESCs deficient for both Suv39h1/2 H3K9 HMTs display similar nucleolar defects. In contrast, Suv4-20h1/2 mutant ESCs lacking H4K20me3 at PCH do not. Suv39h1/2 and Hp1α/β deficiency-induced nucleolar defects are reminiscent of those defining human ribosomopathy disorders. Our results reveal a novel role for SUV39H/HP1-marked repressive constitutive heterochromatin in regulating integrity, function and physiology of nucleoli.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Frottin F., Schueder F., Tiwary S., Gupta R., Körner R., Schlichthaerle T., Cox J., Jungmann R., Hartl F.U., Hipp M.S.. The nucleolus functions as a phase-separated protein quality control compartment. Science. 2019; 365:342–347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

